Back to Journals » Infection and Drug Resistance » Volume 15
A Case of Forearm Soft Tissue Infection Caused by Hypervirulent K. pneumoniae in an Otherwise Healthy 24-Year-Old Woman
Authors Kong L, Wang Y, Ji H, Li Z , Sun Y , Liu Y, Cao S, Zhao J, Shi L, Jin Y
Received 19 October 2021
Accepted for publication 10 December 2021
Published 11 January 2022 Volume 2022:15 Pages 63—68
DOI https://doi.org/10.2147/IDR.S342019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Lingwei Kong,1,* Yu Wang,1,* Hairu Ji,2 Zhehong Li,1 Yupeng Sun,1 Yanchao Liu,1 Sheng Cao,1 Jingxin Zhao,1 Litao Shi,1 Yu Jin1
1Department of Orthopedics, The Affiliated Hospital of Chengde Medical College, Chengde, Hebei, 067000, People’s Republic of China; 2Pathology Teaching and Research Section, Chengde Medical College, Chengde, Hebei, 067000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu Jin Tel +86 13832428328
Email [email protected]
Abstract: In recent years, hypervirulent Klebsiella pneumoniae (hvKp) has received greater attention. It mainly infects diabetic patients and typically causes a hepatic abscess. Here, we report a case of hvKp that caused forearm muscle and soft tissue infection in addition to bacteremia, hepatic and pulmonary abscess, and hyperglycemia. The patient’s condition stabilized after comprehensive treatment. She eventually recovered and was discharged after several debridement and flap operations. At 9 months of follow-up, no signs of infectious recurrence were noted, and the hyperglycemia resolved. Here, we detail important clinical features of a severe hvKp case diagnosed in an otherwise healthy individual. This report underscores the potential of hvKp to cause deep tissue infection and present with clinical symptoms similar to gas gangrene. Symptom onset in the setting of hvKp infection is usually gradual and misdiagnosis is common. The diagnosis of hvKp should be routinely considered in the clinical setting, and be strongly suspected when presenting with characteristic epidemiological, clinical and laboratory features. Although diabetes is a predisposing factor to hvKp infection, hyperglycemia appeared to manifest as a consequence of hvKp infection in this patient.
Keywords: hypervirulent K. pneumoniae, hvKp, soft tissue infection, liver abscess, lung abscess, diabetes
Introduction
Skin and soft tissue infections (SSTI) are infections caused by pathogenic bacteria that penetrate the epidermis, dermis, and subcutaneous tissues. These complex conditions are frequently life-threatening and present with a wide range of clinical symptoms. Patients with tumors, AIDS, diabetes and long-term use of glucocorticoids or immunosuppressants are prone to develop SSTI. The common causative pathogens include Staphylococcus spp., Streptococcus spp. and Clostridium difficile.
Hypervirulent K. pneumoniae (hvKp), first identified in Taiwan in 1986, belongs to the family Enterobacteriaceae and is increasingly seen worldwide.1 Pyogenic liver abscess formation is the primary symptom of hvKp infection, and most patients are diabetic. The string test was used to evaluate hypermucoviscosity2,3 and analyze for the presence of peg-344, iroB, iucA, prmpA, and prmpA2 was performed.4 To the best of our knowledge, this is the first reported clinical case of severe hvKp infection diagnosed in an otherwise healthy individual.
Case Presentation
A 24-year-old woman was admitted to the emergency ward of our hospital complaining of right wrist pain for 7 days in addition to irritation and 1 day of fever. She reported no evident symptoms including edema and erythema in the week preceding the onset of her pain. Since the onset of her pain, the patient visited multiple hospitals and was treated symptomatically, but her condition gradually deteriorated. On presentation to our hospital, right forearm erythema, fever, nausea, vomiting, and chest tightness were noted. Skin integrity was not compromised on her admission to the emergency ward. Although the patient was healthy and had no history of diabetes, she reported suffering an upper respiratory tract infection 2 weeks prior; no particular treatment was provided and her symptoms resolved on their own. On hospital admission, the patient was oriented and the acute face was seen. Physical examination of the chest and abdomen were unremarkable. Marked edema and erythema of the right forearm were apparent, although skin integrity was not compromised. Elevated skin temperature at the site of the lesion, pain on application of pressure and palpable crepitus were noted. The patient had decreased right wrist range of motion due to pain, coolness of the skin distal to the lesion and disturbances in skin sensation; ulnar and radial pulses were palpable (Figure 1A and B). Her temperature, heart rate and blood pressure on admission were recorded to be 37.8°C, 130 beats per minute and 102/70 mmHg, respectively. Laboratory investigations were as follows: peripheral white blood cell count, 17.2×109/L; C-reactive protein, 259 mg/L; procalcitonin, 3.69 ng/mL; ESR, 99 mm/1h; lactate, 2.8 mm/L; creatine kinase, 450 u/L; venous blood glucose, 21 mmol/L. Forearm MRI findings revealed heterogeneous high signal intensity that suggested marked muscular and subcutaneous fatty tissue swelling near the elbow joint distally at the end of the right upper arm and forearm. Multiple segments of soft tissue in the right forearm also exhibited low-signal shadows, which were considered to signify foci of infection (Figure 1C and D). Blood cultures were collected from two sites prior to initiation of antibiotics. A needle aspiration bacterial smear revealed the presence of gram-negative bacteria.
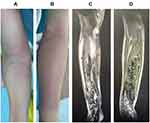 |
Figure 1 (A and B) Redness and swelling of the forearm. (C and D) MRI revealed marked forearm muscle swelling, heterogeneous signal intensity and shadowing suggestive of gas. |
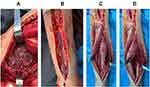
Hypervirulent K. pneumoniae Produced Muscle and Soft Tissue Infection of the Forearm with Bacteremia, Hyperglycemia and Hepatic and Pulmonary Abscesses
The patient’s condition rapidly deteriorated. Her blood pressure decreased, heart rate increased, and forearm tension increased. The range of erythema and edema gradually increased as well. Sensory abnormalities apparent in the hand and other symptoms of compartment syndrome became more prominent. Physical examination was remarkable for palpable crepitus, and gas gangrene could not be ruled out; incisional debridement of the right forearm was subsequently performed. During surgery, necrosis of overlying skin and all pronator muscles was noted. A small amount of pus at the proximal end of the muscular space and large amounts of pus at the distal end of the wrist joint and at the extensor pollicis longus and extensor pollicis brevis between the extensor finger and interosseous membrane were observed (Figure 2A and B). Samples of pus were collected and sent for bacteriological examination. After debridement (Figure 2C and D), the patient was admitted to ICU for further treatment. On the day following surgery, the patient’s temperature remained over 39.0°C, while blood pressure remained less than 80/40 mmHg despite intensive fluid therapy. Blood oxygen level steadily decreased and a diagnosis of septic shock was established. The patient was subsequently ventilated and norepinephrine was administered to improve blood pressure. As gas gangrene could not be excluded, the patient was administered penicillin; meropenem was also administered intravenously for gram-negative coverage. After surgery, the patient was diagnosed with diabetic ketosis after laboratory results revealed repeat random blood glucose >10 mmol/L, glycosylated serum albumin >10.10 g/L, urine glucose + 4 and urine ketones + 3. To maintain glycemic stability, insulin was administered. Gram-negative bacilli were found on blood culture and in pus samples; blood from each culture bottle was inoculated onto blood, MacConkey and chocolate agar plates and incubated at 36°C for 18–24 h. Colony morphology revealed high mucilage content (Figure 3A). Bacterial and molecular analysis revealed expression of rmpA2, rmpA, Peg-344, iucA and iroB virulence genes (Figure 3B). The string test was used to assess hypermucoviscosity using an inoculation loop; formation of mucoviscous strings greater than 5 mm in length was considered a positive result.5 The bacterium was identified to be hvKp using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS; bioMérieux, France). Capsular type (K1, K2, K5, K20, K54, and K57) and relevant virulence (rmpA, rmpA2, iucA, iroB and peg-344) genes were amplified via PCR as previously described.6 Table 1 lists primers used in this study. Agarose gel electrophoresis (AGE) was used to analyze PCR products. Laboratory findings revealed that the strain carried virulence genes rmpA, rmpA2, iucA, iroB, and PEG-344, and was identified to express the K1 capsular serotype (Figure 3C). Bacterial isolation from blood, pus and sputum yielded consistent findings. Bacterial antimicrobial susceptibility testing was performed using Vitek 2 Compact (bioMérieux) GN-09 cards. Antimicrobial susceptibility testing revealed the bacteria to be sensitive to aminoglycoside (eg, gentamicin, tobramycin, amikacin), β-lactam (eg, piperacillin, ceftriaxone, meropenem), quinolone (eg, levofloxacin) and tetracycline (eg, tigecycline) antibiotics. Pulmonary and hepatic abscesses were diagnosed on chest (Figure 4A) and abdominal (Figure 4B) CT; ultrasound-guided drainage was also performed. The patient’s condition stabilized after intensive treatment and she recovered after several debridement and flap operations. At 9 months of follow-up, no infectious recurrence affecting any of the previously infected organs was noted and her hyperglycemia had resolved.
 |
Table 1 List of Primers Used for Detection of Hypervirulent K. pneumoniae Virulence Genes |
 |
Figure 4 (A) Chest CT showing a lung abscess. (B) Abdominal CT showing a multilocular liver abscess. |
Discussion
The main pathogenic bacteria causing suppurative soft tissue infection are Staphylococcus aureus (S. aureus) and Streptococcus pyogenes (S. pyogenes). When infection develops rapidly and manifests with symptoms such as necrotizing fasciitis or myositis, the commonest aerobic bacteria responsible are typically S. pyogenes followed by S. aureus.7 Gas gangrene is seen in the setting of Clostridium difficile (C. difficile).8 A gram-positive coccus naturally present in the normal human microbiota, S. aureus is frequently found on the nasal mucosa, the skin (especially of the axilla and groin) and in the gastrointestinal tract. It is traditionally associated with pus-producing lesions such as furuncles, abscesses and carbuncles. The gram-positive coccus S. pyogenes is also part of the normal human skin microbiota. In contrast to S aureus, the absence of purulence is a distinguishing clinical characteristic of SSTI caused by S. pyogenes, albeit serous fluid-filled blisters can be detected when inflammation is severe. It remains one of the most prevalent causes of nonpurulent cellulitis, causing a variety of clinical infections such as impetigo, lymphangitis, erysipelas, cellulitis and necrotizing fasciitis.9 Clostridium difficile (C. difficile) is a Gram-positive, spore-forming anaerobic bacillus, which is widely distributed in the intestinal tract of humans and animals as well as the general environment. Various types of SSTI ranging from cellulitis to life-threatening gas gangrene and bacteremia may be caused by C. difficile infection. Spontaneous gas gangrene caused by C. difficile is insidious in onset and typically presents with the sole symptom of nonspecific pain in the affected area that is often described as throbbing or heavy. Palpable crepitus, a classic sign of clostridial gas gangrene, usually manifests later.10 The hvKp isolate is a strain of the gram-negative K. pneumoniae. Unlike K. pneumoniae that mainly affects immunocompromised, hospitalized patients, hvKp frequently infects otherwise younger, healthy individuals in the community. Moreover, it can lead to infectious seeding of other tissues, resulting in splenic abscesses, lung abscesses, endophthalmitis and meningitis.3 Necrotizing fasciitis caused by hvKp affecting the musculoperiosteal junction has been rarely reported as a presenting symptom in severe cases.11 In this case, emergency clinical examination could not rule out the presence of gas gangrene; gas detected during surgery may have been produced in the anaerobic environment of the abscess and due to glucose fermentation by hvKp in infected tissue.12 As deep tissue infection by hvKp can result in clinical manifestations similar to gas gangrene, the correct diagnosis may be easily missed. The presence of hvKp should thus be evaluated for in the microbiological diagnosis of community infections and this bacterial infection should be suspected in the setting of suggestive epidemiological, clinical and laboratory features. We recommend sending matching samples for microbiological analysis promptly prior to antibiotic administration so that pathogens may be detected as early as possible in general bacterial smears and cultures.
Diabetes is the most prevalent underlying condition that is a risk factor for hvKp infection; 76.3% of hepatic abscesses due to hvKp infection form in diabetics.13 Higher glucose levels impair neutrophil adhesion, chemotaxis, phagocytosis and bactericidal activity, likewise selectively impairing phagocytosis of hvKp K1/K2 serotypes.14 The patient in this case, however, had no prior history of diabetes and her blood glucose levels increased only after infection with hvKp, remaining elevated throughout the course of infection. The patient’s hyperglycemia resolved on its own 3 months after discharge. As such, hvKp infection appeared to induce hyperglycemia in this patient.
Conclusion
Here, we describe a severe clinical case of hvKp infection diagnosed in an otherwise healthy individual that presented with forearm pain and progressed to include manifestations such as hepatic and pulmonary abscesses as well as hyperglycemia. Thus, it is important to consider the diagnosis of hvKp infection in the setting of limb symptoms mimicking those of gas gangrene. The possibility of infectious seeding to the liver, lungs and other organs should also be considered to avoid misdiagnosis. Although diabetics are more vulnerable to infection with hvKp, hyperglycemia (presumably induced by bacterial damage to islet cells) was noted in this case after hvKp infection. The association between hvKp infection and hyperglycemia warrants further investigation.
Abbreviations
hvKp, hypervirulent K. pneumoniae; CKp, common K. pneumoniae; S. aureus, Staphylococcus aureus; S. pyogenes, Streptococcus pyogenes; PCR, polymerase chain reaction; AGE, agarose gel electrophoresis; AST, antimicrobial susceptibility testing; SSTI, skin and soft tissue infections.
Disclosure
The authors report no conflicts of interest.
References
1. Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146(10):1913–1916. doi:10.1001/archinte.1986.00360220057011
2. Shon AS, Bajwa R, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107–118. doi:10.4161/viru.22718
3. Walkty A, Alexander D, Embil J. An 82-year-old male with a liver abscess. Open Forum Infect Dis. 2020;7(9):ofaa336. doi:10.1093/ofid/ofaa336
4. Russo TA, Olson R, Fang CT, et al. Identification of biomarkers for the differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol. 2018;56(9):
5. Fang CT, Chuang YP, Shun CT, et al. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2004;199(5):697–705. doi:10.1084/jem.20030857
6. Liao W, Long D, Huang Q, et al. Rapid detection to differentiate hypervirulent Klebsiella pneumoniae (hvKp) from classical K. pneumoniae by identifying peg-344 with loop-mediated isothermal amplification (LAMP). Front Microbiol. 2020;11:1189. doi:10.3389/fmicb.2020.01189
7. Barupal SR, Soni ML, Barupal R. Factors affecting mortality following necrotizing soft-tissue infections: randomized prospective study. J Emerg Trauma Shock. 2019;12(2):108. doi:10.4103/JETS.JETS_17_18
8. Junior C, Silva R, Lobato F, et al. Gas gangrene in mammals: a review. J Vet Diagn Investig. 2020;32(2):175–183. doi:10.1177/1040638720905830
9. Breyre A, Frazee BW. Skin and soft tissue infections in the emergency department. Emerg Med Clin North Am. 2018;36(4):723–750. doi:10.1016/j.emc.2018.06.005
10. Srivastava I, Aldape MJ, Bryant AE, Stevens DL. Spontaneous C. septicum gas gangrene: a literature review. Anaerobe. 2017;48:165–171.
11. Prokesch BC, Tekippe M, Kim J, et al. Primary osteomyelitis caused by hypervirulent Klebsiella pneumoniae. Lancet Infect Dis. 2016;16:e190–e195.
12. Law ST, Li M. Is there any difference in pyogenic liver abscess caused by Streptococcus milleri and Klebsiella spp.?: retrospective analysis over a 10-year period in a regional hospital. Immunol Infect. 2013;46(1):11–18. doi:10.1016/j.jmii.2011.12.028
13. Liu C, Guo J. Hypervirulent Klebsiella pneumoniae (hypermucoviscous and aerobactin positive) infection over 6 years in the elderly in China: antimicrobial resistance patterns, molecular epidemiology and risk factor. Ann Clin Microbiol Antimicrob. 2019;18:1.
14. Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metabol. 2006;91(8):3084. doi:10.1210/jc.2005-2749
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.