Back to Journals » Journal of Asthma and Allergy » Volume 14
A Case of Anaphylaxis Caused by Major Royal Jelly Protein 3 of Royal Jelly and Its Cross-Reactivity with Honeycomb
Authors Li JD , Cui L, Xu YY , Guan K
Received 29 October 2021
Accepted for publication 14 December 2021
Published 22 December 2021 Volume 2021:14 Pages 1555—1557
DOI https://doi.org/10.2147/JAA.S346045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Jun-Da Li,1– 3 Le Cui,1– 3 Ying-Yang Xu,1– 3 Kai Guan1– 3
1Department of Allergy, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China; 2Beijing Key Laboratory of Precision Medicine for Diagnosis and Treatment on Allergic Diseases, Beijing, People’s Republic of China; 3National Clinical Research Center for Dermatologic and Immunologic Disease, Beijing, People’s Republic of China
Correspondence: Kai Guan
Department of Allergy, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, #1 Shuaifuyuan, Wangfujing, Beijing, 100730, People’s Republic of China
Tel/Fax +86 10-69156346
Email [email protected]
Purpose: Royal jelly and honeycomb are commonly consumed in China, and anaphylaxis caused by ingestion of royal jelly is rare. To date, there is no report of anaphylaxis after ingestion of royal jelly in China. Its cross-reactivity with honeycomb is still unclear.
Case Report: A 56-year-old Chinese female experienced two episodes of anaphylaxis within 1 hour after ingestion of royal jelly within one month. After avoiding royal jelly and other bee products, no anaphylactic reaction occurred again. The skin prick test and basophil activation test showed positive reactivity to royal jelly and honeycomb. In immunoblotting and immunoblotting inhibition tests, a 60 kDa protein was recognized in royal jelly and cross-reactivity with honeycomb. The mass spectrometry data revealed that the 62kDa protein belongs to major royal jelly protein 3.
Conclusion: Our data suggest that major royal jelly protein 3 of royal jelly is a main allergen that induces anaphylaxis and cross-reactivity with honeycomb. Therefore, the patient was allergic to royal jelly to avoid other bee products.
Keywords: royal jelly, honeycomb, major royal jelly protein 3, anaphylaxis
Introduction
Royal jelly is secretions from the hypopharyngeal and mandibular glands of worker bees.1,2 Honeycombs are dwelling places where honeybees live and breed. It is thought to improve health status and promote immune function, so it is widely consumed in China as a health tonic or alternative medicine. Although a few cases3–5 of anaphylaxis due to royal jelly were reported in Japan, this is the first Chinese report of royal jelly-induced anaphylaxis. Many proteins contained in royal jelly can induce anaphylaxis. We identified 62 kDa major royal protein 3 which was the main allergen in our cases and cross-reactivity with honeycomb by several experiments.
Case Presentation
A 56-year-old Chinese female visited the Department of Allergy, Peking Union Medical College Hospital because he experienced two episodes of anaphylaxis within 1 hour after ingestion of food within one month. The clinical syndrome included urticaria, pruritus, laryngeal edema, chest tightness, hypotension, and collapse.
Skin prick tests were performed with crude extracts of royal jelly, honey, and honeycomb from our laboratory. Skin prick tests revealed that royal jelly (4 mmx4 mm wheal and 37 mm x 16 mm erythematous area) and honeycomb (3 mmx3 mm wheal and 36 mmx 20mm erythematous area) positive, honey was negative. The total IgE was 438kU/L, specific IgE antibodies to i1 (0.19kUA/L), i3 (0.03kUA/L), i208 (0.01kUA/L) and i209 (0.01kUA/L) were below 0.35kUA/L by ImmunoCAP (Thermofisher, USA).
The basophil activation test was analysed by the Flow CAST kit (BÜHLMANN, Schönenbuch, Switzerland), as shown in Figure 1. According to the manufacturer’ s protocol, coincubation with whole peripheral blood and crude extract of royal jelly and honeycomb respectively (0.25 µg /mL, 0.5 µg /mL and 1 µg /mL), CCR3+CD63+cells were defined as activated basophils that were determined by flow cytometry (Partec, Germany) according to the manufacturer’s protocol. For the crude extract of RJ, 4.53%, 6.9%, and 9.58% of activated basophils were detected, 0.64%, 1.18%, and 2.81% of activated basophils were detected when the crude extract of honeycomb as a stimulant.
 |
Figure 1 The results of basophil activation test. |
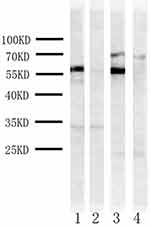
The proteins of bee venom, royal jelly and honeycomb were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting analysis of royal jelly extract (Figure 2) revealed serum IgE antibodies reacting only 60 kDa protein. For honeycomb extract, serum IgE antibodies bound to 70 kDa protein and 60 kDa protein. No immunoreactive bands were detected in bee venom extract. In the immunoblotting inhibition test, honeycomb extract showed complete inhibition of IgE, which can bind to royal jelly 60 kDa protein, and the same inhibition occurred when royal jelly extract was an inhibitor.
We extracted 60 kDa and 70 kDa proteins from polyacrylamide gels and analyzed them by high-performance liquid chromatography–mass spectrometry (HPLC–MS) method. According to the mass spectrometry data, 60 kDa protein had 18 unique peptide and 34 unique spectrum of 62kDa major royal jelly protein 3, 70 kDa protein had 4 unique peptide and 4 unique spectrum of 70kDa major royal jelly protein 5.
Discussion and Conclusion
In our study, we identified that the culprit protein of royal jelly is 62 kDa-major royal jelly protein 3 and showed cross-reactivity with other bee product honeycombs. The skin prick test and basophil activation test confirmed allergens in vitro and in vivo, respectively, and basophils showed higher reactivity to royal jelly extract than honeycomb. Serum IgE antibody specific binding to 60 kDa protein was simultaneously discovered in royal jelly and honeycomb, band of 70 kDa protein only shown in honeycomb which is weaker than band of 60 kDa protein. In the immunoblotting inhibition test, serum IgE-binding to 60 kDa protein was inhibited by both extractions, which suggested that there were some similar proteins in royal jelly and honeycomb. According to mass spectrometry data, there was a very higher protein identification probability to identify 62 kDa MRJP 3 and 70 kDa MRJP 5,7 and to have more than 60% similarity amino acid sequences through alignment.
Bee products are favored by customers because of their health benefits in China. Food-induced anaphylaxis caused by ingestion of royal jelly is rare, and a few cases6,8 have been reported overseas, most in Japan. This is the first case of anaphylaxis reported in China. In 2011, Mizutani et al4 reported a case of anaphylaxis caused by ingestion of royal jelly and deduced that major royal jelly protein37 is a possible allergen. Our study proved that various bee product could induce anaphylaxis and that it has nothing to do with bee venom, allergenic cross-reactivity between royal jelly and honeycomb was found. Therefore, patients who are allergic to royal jelly should avoid other bee products.
Declaration of Patient Consent
The authors obtained informed consent for publication from the patient. The patient understands her personal information will not be published. The case required institutional approval to publish the case details and was reviewed and approved by the Ethical Committee of Peking Union Medical College.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Beijing Natural Science Foundation Project (7172179).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hata T, Furusawa T, Arai Y, et al. Studies of royal jelly and associated cross-reactive allergens in atopic dermatitis patients. PLoS One. 2020;15(6):e0233707. doi:10.1371/journal.pone.0233707
2. Pasupuleti VR, Sammugam L, Ramesh N, Gan SH. Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxid Med Cell Longev. 2017;2017:1259510. doi:10.1155/2017/1259510
3. Katayama M, Aoki M, Kawana S. Case of anaphylaxis caused by ingestion of royal jelly. J Dermatol. 2008;35(4):222–224. doi:10.1111/j.1346-8138.2008.00448.x
4. Mizutani Y, Shibuya Y, Takahashi T, et al. Major royal jelly protein 3 as a possible allergen in royal jelly-induced anaphylaxis. J Dermatol. 2011;38(11):1079–1081. doi:10.1111/j.1346-8138.2010.01179.x
5. Takahama H, Shimazu T. Food-induced anaphylaxis caused by ingestion of royal jelly. J Dermatol. 2006;33(6):424–426. doi:10.1111/j.1346-8138.2006.00100.x
6. Leung R, Thien FC, Baldo B, et al. Royal jelly-induced asthma and anaphylaxis: clinical characteristics and immunologic correlations. J Allergy Clin Immunol. 1995;96(6 Pt 1):1004. doi:10.1016/S0091-6749(95)70242-3
7. Matuszewska E, Matysiak J, Rosiński G, et al. Mining the royal jelly proteins: combinatorial hexapeptide ligand library significantly improves the MS-based proteomic identification in complex biological samples. Molecules. 2021;26(9):2762. doi:10.3390/molecules26092762
8. Thien FC, Leung R, Baldo BA, et al. Asthma and anaphylaxis induced by royal jelly. Clin Exp Allergy. 1996;26(2):216–222. doi:10.1111/j.1365-2222.1996.tb00082.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.