Back to Journals » Cancer Management and Research » Volume 13
A Case of Acute Myeloid Leukemia Harboring a Rare Three-Way Translocation t(5;7;7) Involving the PDGFRB Gene and Successfully Treated with Imatinib
Authors Borogovac A , Sahu KK , Vishwanathan GK , Miron PM, Cerny J
Received 25 August 2021
Accepted for publication 22 October 2021
Published 25 November 2021 Volume 2021:13 Pages 8841—8847
DOI https://doi.org/10.2147/CMAR.S324718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Azra Borogovac,1 Kamal Kant Sahu,2 Ganesh Kumar Vishwanathan,3 Patricia Minehart Miron,4 Jan Cerny5
1Hematology‐Oncology Section, Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA; 2Division of Hematology and Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA; 3Department of Hematology, All India Institute of Medical Sciences (AIIMS), New Delhi, India; 4Department of Pathology, University of Massachusetts Memorial Medical Center, Worcester, MA, USA; 5Division of Hematology and Oncology, Department of Medicine, University of Massachusetts Memorial Medical Center, Worcester, MA, USA
Correspondence: Jan Cerny
Division of Hematology and Oncology, Department of Medicine, University of Massachusetts Memorial Medical Center, Worcester, MA, USA
Email [email protected]
Abstract: Platelet-derived growth factor-beta (PDGFRB) gene maps for the receptor tyrosine kinase PDGRFβ. PDGFRB gene fusions have been implicated in multiple myeloid and lymphoid neoplasms and have shown exquisite sensitivity to tyrosine kinase inhibitors. We report a case of a 29-year-old male who presented with acute myeloid leukemia who was eventually found to harbor a unique three-way translocation t(5;7; 7)(q33.2;q32;q11.2) involving the PDGFRB gene. The patient initially achieved a complete response after induction with daunorubicin and cytarabine, but when he returned for consolidation, his white cell count had increased, and he was found to have an underlying myeloproliferative neoplasm. He was given consolidation with high-dose cytarabine and imatinib with excellent response, and ultimately received a matched unrelated donor transplant. The patient remains in remission to this day more than eight years later.
Keywords: AML, eosinophilia, PDGFRB, translocation, imatinib, karyotype
Introduction
Platelet-derived growth factor (PDGF) is a dimeric protein composed of two receptor tyrosine kinases, α and β, encoded by the PDGFRA (4q12) and PDGFRB (5q32) genes.1 PDGFRB gene rearrangements create chimeric proteins that can cause constitutive tyrosine kinase activity, which leads to upregulation of transcription factors affecting cell growth and survival.2,3 The first described, and most common PDGFRB rearrangement, is the (5;12)(q33;p13) translocation with ETV6; however, approximately 30 other different fusion partners of PDGFRB have been identified to date.4–8
Myeloid and lymphoid neoplasms with eosinophilia associated with PDGFR or fibroblast growth factor receptor 1 (FGFR-1) rearrangements are grouped into their own clinical entity according to the World Health Organization (WHO) classification, although eosinophilia may be absent in a subset of cases with these rearrangements.9 The PDGFRB fusion genes have been reported in all major groups of myeloid neoplasms including myelodysplastic syndrome (MDS)/myeloproliferative neoplasms (MPN), acute myeloid leukemia (AML), and in lymphoid neoplasms, such as B-cell and T-cell acute lymphoblastic leukemias (ALL).5,6,10–12 Among patients with MPNs, PDGFRB rearrangement is seen in approximately 2% of patients and is associated with leukocytosis, eosinophilia and anemia.13 The presence of PDGFRB rearrangement is associated with excellent response to imatinib, including in patients that have failed standard chemotherapy.4,14–18
We report a case of a 29-year-old man who initially presented with AML and achieved a complete response (CR) after induction chemotherapy. However, when he was admitted for consolidation chemotherapy, he was found to have an elevated white cell count of 35,000/mm3, for which a repeat bone marrow biopsy with cytogenetic analysis was performed. He was subsequently found to have an MPN harboring a unique three-way translocation t(5;7;7)(q33.2;q32;q11.2) involving the PDGRB gene. The patient achieved a good response with the addition of imatinib to his consolidation chemotherapy and subsequently underwent an allogeneic stem cell transplant (alloSCT).
Case Presentation
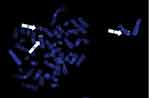
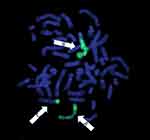
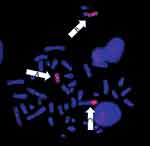
Our patient was a 29-year-old male who presented with loss of vision in the right eye. He also reported fatigue, dyspnea, night sweats and left upper quadrant fullness for approximately one month before presentation. His initial complete blood count revealed a total leukocyte count of 380,000/mm3 with 66% blasts, 5% segmented neutrophils, 8% monocytes, 3% eosinophils, and other immature myeloid cells (Table 1). He was anemic and thrombocytopenic with a hemoglobin of 7.8 gm/dL and a platelet count of 35,000/mm3. A bone marrow biopsy was a diagnostic of AML with monocytic differentiation. There were no FLT3 nor NPM1c terminal mutations. Cytogenetic analysis showed a 3-way translocation involving chromosome 5 and both chromosomes 7 with breakpoints at 5q33.2, 7q32 and 7q11.2 [(46, XY,t(5;7;7)(q33.2;q32;q11.2)] as the only abnormality (Figure 1). Interphase fluorescence in situ hybridization (FISH) was positive for PDGFRB rearrangement. Metaphase FISH with PDGFRB and with whole chromosome painting probes to chromosomes 5 and 7 confirmed the 3-way translocation and showed that sequences 5ʹ to PDGFRB were translocated to the derivative chromosome 7 with the 7q32 breakpoint (Figure 2). For other PDGFRB fusions, the critical oncogenic fusion is on the derivative chromosome 5, which retains the 3ʹ portion of PDGFRB (Figure 3). Thus, the partner gene for this unidentified PDGFRB rearrangement appeared to lie at the 7q11.2 breakpoint (Figure 4). BCR-ABL1 fusion was not observed. The patient was started on induction chemotherapy with cytarabine and daunorubicin (7+3). Bone marrow biopsies on days 14 and 28 were negative for AML consistent with a successful induction.
 |
Table 1 Peripheral Blood and Bone Marrow Trends Throughout Treatment Course |
 |
Figure 1 Karyotype. 46,XY,t(5;7;7) (q33.2; q32; q11.2). Arrows indicate the direction of the transfer of chromosomal material between the derivative chromosomes 5, 7 and 7. |
When he presented for consolidation chemotherapy, the patient was found to have a total white blood cell count of 35,000/mm3 with 50% segmented neutrophils, 21% monocytes, and no blasts. Due to concern for disease relapse, a repeat bone marrow biopsy was performed which demonstrated 95% hypercellular marrow with granulocytic hyperplasia, monocytosis, eosinophilia, and megakaryocytic hypoplasia. Morphologically, the bone marrow was consistent with chronic myelomonocytic leukemia (CMML) with eosinophilia of 7%. Eosinophilia was not present in the peripheral blood. The patient had persistence of the same clonal rearrangement. The patient proceeded with high-dose ARA-C (HiDAC) for consolidation. Due to the PDGFRB translocation, imatinib 400 mg daily was added to his consolidation regimen.
A repeat bone marrow one month after initiation of imatinib therapy demonstrated continued morphological remission but persistent PDGFRB rearrangement. He subsequently underwent a matched unrelated donor alloSCT 3 months after initiation of imatinib with myeloablative conditioning. He has remained in a clinical and pathological CR 8 years later.
Discussion
We report a patient with a rare translocation involving the PDGFRB gene, who was initially treated with induction 7+3 chemotherapy for AML and was subsequently found to have an underlying MPN. Metaphase chromosome analysis demonstrated a three-way translocation (5;7;7)(q33.2;q32;q11.2); FISH confirmed the involvement of the PDGFRB gene. To the best of our knowledge, this is the first such reported case of a PDGFRB translocation with a fusion product that would be equivalent to that generated by a more straight-forward two-way translocation, t(5;7;7)(q33.2;q11.2). The patient was treated with HiDAC consolidation chemotherapy for the AML along with targeted therapy with imatinib. He maintained his counts for 3 months until alloSCT and remains in remission to this date. As in our case, the PDGFRB translocation in acute leukemia is often not found until the patient experiences relapse.19 Additionally, the diagnosis may be more difficult if peripheral eosinophilia is not present. In one study, approximately 4 of 19 patients (21%) with hematological malignancies with PDGFRB rearrangements did not have eosinophilia and only 11/19 (58%) of cases had eosinophils ≥ 1.5 × 109/L.7
The mechanism by which PDGFR fusion proteins lead to malignant neoplasms is thought to occur through constitutive activation of the PDGFRs. Normally, the PDGF proteins assemble causing dimerization of the PDGFRs, which activate the cell’s tyrosine kinase activity. This sequence of events leads to downstream signaling cascades important in cell migration, proliferation and survival through many overlapping signaling pathways.2,20 One such pathway activated by the PDGFR is the PI3K/AKT/mTOR pathway, which is frequently dysregulated in many cancers.21
Identifying the PDGFRB rearrangement in hematological malignancies early in disease has important treatment implications as they have been shown to respond favorably to tyrosine kinase inhibitors (TKIs). In a cohort of 26 patients with hematological malignancies with PDGFRB translocations treated with imatinib therapy, Cheah et al demonstrated a 10-year overall survival (OS) of 90% after a median duration of imatinib of 6.6 years, and the 6-year progression-free survival (PFS) of 88%. No patients developed treatment resistance; furthermore, all patients that achieved hematological response did so within 2 months.4 Jawhar et al also evaluated 22 patients with PDGFRB translocations with myeloid and lymphoid malignancies (17 patients in chronic phase, and 5 patients in blastic phase) and found all the chronic phase patients achieved a CR with imatinib alone. The 5 patients in blastic phase (2 myeloid, and 3 lymphoid) were treated with a combination of intensive chemotherapy and imatinib, consolidated with alloSCT for 3 of the patients. The 5-year OS was 50% in those who presented in blastic phase versus 92% in chronic phase.7
It is important to note that only a few cases have been reported with AML involving the PDGFRB gene, and the response to TKIs is not as clearly established. In a review of case reports, Naymagon et al documented 4 cases of AML associated with PDGFRB translocations.19 Three cases resulted from de novo AML, while one had an underlying MPN. All four cases achieved a CR with induction chemotherapy and imatinib, but only 2 of 4 maintained a CR after 12 months or more of follow-up.19 Tokita et al reported a patient with chronic idiopathic myelofibrosis with TEL-PDGFRB fusion that transformed into AML. The patient was similarly treated with induction chemotherapy and imatinib. The patient responded well for three months until his AML relapsed, and he died shortly thereafter.22 Shimomura et al reported the first case of AML with PDGFRB rearrangement to be treated with single-agent imatinib for induction therapy and achieved hematological remission; however, his AML shortly relapsed. They hypothesized treatment resistance was due to a point mutation in the PDGFRB gene.16 Several PDGFRB rearrangements have also been associated with resistance to imatinib and other TKIs.23–25 No studies have reported the role of later generation TKIs in the setting of AML with PDGFRB rearrangements after resistance to imatinib.
Conclusion
The PDGFRB rearrangement can present as a wide array of hematological malignancies. Our patient’s AML responded to induction chemotherapy; however, his MPN surfaced once he achieved remission for AML. He subsequently achieved a hematological response after initiation of imatinib, which he maintained until transplantation; as such, it is unclear how he would have responded with long-term tyrosine kinase inhibition. This case further argues for early cytogenetic analysis of PDGFR rearrangements in patients with a diverse presentation of hematological malignancies.
IRB Approval
IRB approval was not required to publish this case report.
Ethical Statement
The article does not contain participation of any human being and animal.
Patient Consent
As an institutional protocol, patient was consented at the beginning of his treatment only that if required his treatment data would be used for publication without revealing his identity. Hence, we confirm that we have taken appropriate consent from the patient, which allows us to report this case.
Funding
There is no funding to report.
Disclosure
Dr. Jan Cerny has the following disclosures outside the published work: he serves in the advisory board/consultancy for Jazz Pharmaceuticals, Pfizer and Amgen. He is a Data and Safety Monitoring Board Member for AlloVir, and holds stocks from Actinium Pharmaceuticals, Bluebird Bio Inc., Dynavax Pharma, Atyr Pharmac, Gamida Cell, Miragen Therapeutics, Mustang Bio, Novavax, Ovid Therapeutics, Sorrento Therapeutics, TG Therapeutics, Vaxart Inc., and Veru Inc. The authors report no other conflicts of interest in this work.
References
1. Demoulin JB, Montano-Almendras CP. Platelet-derived growth factors and their receptors in normal and malignant hematopoiesis. Am J Blood Res. 2012;2:44–56.
2. Kazlauskas A. PDGFs and their receptors. Gene. 2017;614:1–7. doi:10.1016/j.gene.2017.03.003
3. Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77:307–316. doi:10.1016/0092-8674(94)90322-0
4. Cheah CY, Burbury K, Apperley JF, et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood. 2014;123:3574–3577. doi:10.1182/blood-2014-02-555607
5. Vega F, Medeiros LJ, Bueso-Ramos CE, et al. Hematolymphoid neoplasms associated with rearrangements of PDGFRA, PDGFRB, and FGFR1. Am J Clin Pathol. 2015;144:377–392. doi:10.1309/AJCPMORR5Z2IKCEM
6. Appiah-Kubi K, Lan T, Wang Y, et al. Platelet-derived growth factor receptors (PDGFRs) fusion genes involvement in hematological malignancies. Crit Rev Oncol Hematol. 2017;109:20–34. doi:10.1016/j.critrevonc.2016.11.008
7. Jawhar M, Naumann N, Schwaab J, et al. Imatinib in myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRB in chronic or blast phase. Ann Hematol. 2017;96:1463–1470. doi:10.1007/s00277-017-3067-x
8. Reiter A, Gotlib J. Myeloid neoplasms with eosinophilia. Blood. 2017;129:704–714. doi:10.1182/blood-2016-10-695973
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi:10.1182/blood-2016-03-643544
10. Bain BJ. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB or FGFR1. Haematologica. 2010;95:696–698. doi:10.3324/haematol.2009.021675
11. Morerio C, Acquila M, Rosanda C, et al. HCMOGT-1 is a novel fusion partner to PDGFRB in juvenile myelomonocytic leukemia with t(5;17)(q33;p11.2). Cancer Res. 2004;64:2649–2651. doi:10.1158/0008-5472.CAN-03-4026
12. Pozdnyakova O, Orazi A, Kelemen K, et al. Myeloid/lymphoid neoplasms associated with eosinophilia and rearrangements of PDGFRA, PDGFRB, or FGFR1 or with PCM1-JAK2. Am J Clin Pathol. 2021;155:160–178. doi:10.1093/ajcp/aqaa208
13. Arefi M, Garcia JL, Penarrubia MJ, et al. Incidence and clinical characteristics of myeloproliferative neoplasms displaying a PDGFRB rearrangement. Eur J Haematol. 2012;89:37–41. doi:10.1111/j.1600-0609.2012.01799.x
14. Metzgeroth G, Schwaab J, Gosenca D, et al. Long-term follow-up of treatment with imatinib in eosinophilia-associated myeloid/lymphoid neoplasms with PDGFR rearrangements in blast phase. Leukemia. 2013;27:2254–2256. doi:10.1038/leu.2013.129
15. David M, Cross NC, Burgstaller S, et al. Durable responses to imatinib in patients with PDGFRB fusion gene-positive and BCR-ABL-negative chronic myeloproliferative disorders. Blood. 2007;109:61–64. doi:10.1182/blood-2006-05-024828
16. Shimomura Y, Maruoka H, Ishikawa T. Marked response to imatinib mesylate in a patient with platelet-derived growth factor receptor beta-associated acute myeloid leukemia. Int J Hematol. 2017;105:697–701. doi:10.1007/s12185-016-2167-z
17. Bell GC, Padron E. Detection of a PDGFRB fusion in refractory CMML without eosinophilia: a case for broad spectrum tumor profiling. Leuk Res Rep. 2015;4:70–71.
18. Wenger TL, Bly RA, Wu N, et al. Activating variants in PDGFRB result in a spectrum of disorders responsive to imatinib monotherapy. Am J Med Genet A. 2020;182:1576–1591. doi:10.1002/ajmg.a.61615
19. Naymagon L, Marcellino B, Mascarenhas J. Eosinophilia in acute myeloid leukemia: overlooked and underexamined. Blood Rev. 2019;36:23–31. doi:10.1016/j.blre.2019.03.007
20. Wu E, Palmer N, Tian Z, et al. Comprehensive dissection of PDGF-PDGFR signaling pathways in PDGFR genetically defined cells. PLoS One. 2008;3:e3794. doi:10.1371/journal.pone.0003794
21. Zhang H, Bajraszewski N, Wu E, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–738. doi:10.1172/JCI28984
22. Tokita K, Maki K, Tadokoro J, et al. Chronic idiopathic myelofibrosis expressing a novel type of TEL-PDGFRB chimaera responded to imatinib mesylate therapy. Leukemia. 2007;21:190–192. doi:10.1038/sj.leu.2404397
23. Byrgazov K, Kastner R, Gorna M, et al. NDEL1-PDGFRB fusion gene in a myeloid malignancy with eosinophilia associated with resistance to tyrosine kinase inhibitors. Leukemia. 2017;31:237–240. doi:10.1038/leu.2016.250
24. Zhang Y, Gao Y, Zhang H, et al. PDGFRB mutation and tyrosine kinase inhibitor resistance in Ph-like acute lymphoblastic leukemia. Blood. 2018;131:2256–2261. doi:10.1182/blood-2017-11-817510
25. Tran TH, Nguyen JV, Stecula A, et al. The EBF1-PDGFRB T681I mutation is highly resistant to imatinib and dasatinib in vitro and detectable in clinical samples prior to treatment. Haematologica. 2021. doi:10.3324/haematol.2020.261354
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.