Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
A Canadian cost-effectiveness analysis of SAPIEN 3 transcatheter aortic valve implantation compared with surgery, in intermediate and high-risk severe aortic stenosis patients
Authors Tarride JE, Luong T , Goodall G , Burke N , Blackhouse G
Received 9 March 2019
Accepted for publication 16 May 2019
Published 29 July 2019 Volume 2019:11 Pages 477—486
DOI https://doi.org/10.2147/CEOR.S208107
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Jean-Eric Tarride,1–3 Trinh Luong,4 Gordon Goodall,5 Natasha Burke,3 Gordon Blackhouse2,3
1McMaster Chair in Health Technology Management, Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada; 2Center for Health Economics and Policy Analysis (CHEPA), McMaster University, Hamilton ON, Canada; 3Programs for Assessment of Technology in Health (PATH), The Research Institute of St. Joe’s, St Joseph’s Healthcare Hamilton, Hamilton, ON, Canada; 4Edwards Lifesciences (Canada) Inc., Mississauga, ON, Canada; 5Edwards Lifesciences SA, Nyon, Switzerland
Background and objectives: The treatment of severe aortic stenosis requires replacement of the defective native valve. Traditionally, this has been done via surgery, but in the last 10 years, transcatheter techniques have emerged. Transcatheter aortic valve implantation (TAVI) is a less invasive option compared to surgical aortic valve replacement (SAVR), and this study evaluates the cost-effectiveness of TAVI versus SAVR in intermediate and high surgical risk patients in Canada.
Methods: A Markov model was used to project the costs and quality-adjusted life years (QALYs) gained for TAVI using the SAPIEN 3 valve and SAVR over a 15-year time horizon. The PARTNER I and II studies were used to populate the model in terms of survival, clinical event rates and quality of life over time. The costs of TAVI with SAPIEN 3 and SAVR as well as the costs associated with events included in the model were derived from Canadian administrative and literature data. Costs were expressed in 2018 Canadian dollars and all future costs and QALYs were discounted at a rate of 1.5% annually. Probabilistic and one-way sensitivity analyses were conducted.
Results: The incremental cost-effectiveness ratios of TAVI using the SAPIEN 3 valve compared to surgery were $28,154 per QALY gained in intermediate risk patients and $17,237 per QALY gained in high-risk patients. The results of the probabilistic analyses indicated that at willingness-to-pay threshold of $50,000 per QALY gained, the probability of TAVI to be cost-effective was greater than 0.9 in both intermediate-risk and high-risk patients. Sensitivity analyses showed the results were most sensitive to the time horizon used.
Conclusion: TAVI using the SAPIEN 3 valve is highly likely to be cost-effective in Canadian patients with severe aortic stenosis who are at intermediate and high surgical risk.
Keywords: aortic stenosis, economic evaluation, valve replacement, Canada
Introduction
Aortic stenosis is a valvular heart disease that involves the narrowing of the aortic valve. In a recent systematic review, the prevalence of severe aortic stenosis amongst the elderly (>75 years old) was estimated to be 3.4%.1 Severe aortic stenosis is associated with a very high mortality rate if left untreated with approximately 75% of patients likely to die within 3 years of symptoms.2 Surgical aortic valve replacement (SAVR) has been the traditional treatment for severe aortic stenosis, although in many cases SAVR is not performed because patients are being considered inoperable due to frailty, co-morbidities or advanced age.2 In recent years, transcatheter aortic valve implantation (TAVI) has emerged as a less invasive option to treat the disease, and in randomized clinical trials, it has been shown to be superior to best medical treatment3 in inoperable patients and at least noninferior to SAVR in high-risk4,5 and intermediate-risk6,7 patients.
Following the first in man use of TAVI in 2002 and commercialization in 2007, devices have undergone substantial development. The third generation of transcatheter heart valves such as the Edwards Lifesciences SAPIEN 3™ is now commercially available.8 A recent study evaluated outcomes for TAVI using the SAPIEN 3 device in inoperable, high-risk and intermediate-risk patients with severe aortic stenosis. In a propensity score-adjusted analysis, 1-year outcomes for intermediate-risk patients with SAPIEN 3 were compared to SAVR patients in the PARTNER II RCT.9 Mortality at 1 year with SAPIEN 3 was estimated to be 7.4% compared to 13% with SAVR and the 1-year risk of stroke was 4.6% for SAPIEN 3 compared to 8.2% for SAVR. Additionally, a recent economic evaluation in the US of PARTNER IIA and SAPIEN 3 results in intermediate-risk patients found that TAVI dominated SAVR (ie better outcomes and less costly than SAVR).10 In Canada, three recent economic evaluations have shown that TAVI was cost-effective in Canadian intermediate-risk11,12 and high-risk patients.13 However, no Canadian studies have evaluated the cost-effectiveness of TAVI using the SAPIEN 3 heart valve device compared to SAVR in intermediate-risk and high-risk Canadian patients.
Methods
Model overview
An economic model was used to estimate the costs and quality adjusted life years (QALYs) of patients with severe aortic stenosis undergoing either 1) TAVI using the SAPIEN 3 prosthesis or 2) SAVR in Canada. Two different patient populations were evaluated: patients at intermediate and patients at high surgical risk. The analysis was made from a Canadian third-party payer perspective. The model used a 15-year time horizon and a 1.5% discount rate for costs and outcomes (ie QALYs).
Figure 1 presents the structure of the economic model. In each monthly cycle of the model, patients can be in 1 of 9 mutually exclusive health states primarily defined by NYHA (New York Heart Association) class and whether the patient has suffered a stroke during the model. In addition to health states defined by NYHA class and stroke status, patients are also at risk of death every monthly cycle. Patients can transition between any of the non-death health states each cycle and different costs and utility values can be assigned to each of these. Patients are assigned the cost of treatment (SAVR, TAVI) including the device cost and associated hospitalization cost during the first cycle of the model. Thereafter, simulated patients are at risk of acute events each cycle from any of the nondeath health states including stroke, transient ischemic attack (TIA), pacemaker implantation, endocarditis, new-onset atrial fibrillation, myocardial infarction, renal replacement therapy, major bleeding, re-hospitalization and reinterventions with TAVI using SAPIEN 3 or SAVR.
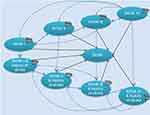 |
Figure 1 Graphical representation of the model structure. Abbreviation: NYHA, New York Heart Association. |
Model inputs
Clinical inputs
The distribution of patients by NYHA class, mortality rates and clinical event rates used in the model was derived from 3 main sources of data: 1) PARTNER IA study14 for the SAVR high-risk population; 2) PARTNER IIA study6 for the SAVR intermediate-risk population and 3) PARTNER II study for TAVI with the SAPIEN 3 valve in intermediate- and high-risk populations.9,15 Tables 1 and 2 present for TAVI using SAPIEN 3 and SAVR the 30-day and 1-year observed event rates for intermediate- and high-risk patients, respectively. For TAVI using SAPIEN 3, outcome data were available up to 12 months for both the intermediate- and the high-risk populations. For SAVR, data on all clinical outcomes was available up to 2 years for intermediate-risk and 5 years for high-risk arms. Mortality rates beyond those observed in the trials were extrapolated and a range of survival statistical functions were tested. Based on goodness of fit tests (Akaike information criterion and Bayesian information criterion) and clinical plausibility comparing predictions to general population mortality data, a linear function and a Weibull distribution were selected to model mortality in the intermediate-risk and high-risk populations, respectively. Trial data and extrapolations based on the last observed data were used for the other clinical events included in the model.
 |
Table 1 Clinical outcomes for intermediate-risk patients |
 |
Table 2 Clinical outcomes for high-risk patients |
Costs input
The cost estimates of TAVI using SAPIEN 3 and SAVR procedures were comprised of various components. These included the cost of the device for each procedure, the post-procedural inpatient costs, physician fees related to the procedure and to specialist consultations during the inpatient stay, along with workup costs that occurred in an emergency room or ambulatory setting just prior to admission. The cost of the TAVI using SAPIEN 3 device was based on the manufacturer list price ($25,000). The cost of the SAVR device ($6,000) was derived from a recent Canadian cost-effectiveness study comparing TAVI to SAVR in intermediate-risk patients.11 Post-procedural inpatient costs (eg hospitalization costs) were calculated using unpublished patient level data from the Canadian Institute of Health Information (CIHI) for more than 8,000 individuals undergoing TAVI or SAVR for severe aortic stenosis between April 1, 2014 and March 31, 2016 (Hamilton Integrated Review Ethics Board approval #2018-3141). Work-up costs were also identified using the same CIHI data. Physician fees associated with SAVR and TAVI using SAPIEN 3 procedures were based on expert opinion and the Ontario Schedule of Benefits for Physician Services.16 Hospitalization costs associated with TAVI and SAVR were estimated separately for intermediate- and high-risk patients. The logistic EuroScore I (log ES),17 which can be approximated using administrative data from CIHI, was used to predict the operative risk group for each patient. High-risk patients were defined as those with a log ES greater or equal to 15% while intermediate-risk patients were defined as those with a log ES score between 5% and less than 15%. Table 3 provides the total and breakdown of procedure-related costs for TAVI with SAPIEN 3 and SAVR used in the model per surgical risk levels. In addition, all TAVI patients as well as SAVR patients with non-mechanical heart valves were assigned costs of 75 mg OD clopidogrel for the first 6 months after their procedure at a cost of $73.18 SAVR patients who received mechanical valves, approximately 15% of all valves, were assumed to receive on average 5.8 mg of warfarin per day for the rest of their lives,19 which was calculated at $278 per year (including drug and INR testing costs16,20). Canadian-specific costs of events were derived from published literature,21 the CIHI patient cost calculator22 and Alberta Health costing23 (Table 4). All costs are presented in 2018 Canadian dollars, and the Canadian Consumer Price Index24 was used when required to inflate costs to 2018 values.
 |
Table 3 Procedural cost by patient risk population* |
 |
Table 4 Costs and dis-utilities applied to model events |
Utilities input
Health state utility values considered in the model were derived from patient-level EQ5D-3L data collected in the PARTNER studies, which were valued using the Canadian EQ5D-3L algorithm.25 For intermediate-risk patients, EQ5D-3L utility values of 0.80 and 0.72 were assigned to TAVI using SAPIEN 3 and SAVR patients for the first year postprocedure. Beyond 1 year, these utility values were 0.79 and 0.78, respectively. EQ-5D-3L utility values for high-risk patients were lower for both the first-year post procedure (ie TAVI with SAPIEN 3: 0.75; SAVR: 0.69) and beyond 1 year (ie TAVI using SAPIEN 3: 0.76; SAVR: 0.74). For patients with stroke, a utility weight of 0.6826 was applied to the procedure and time-specific utility values. In the base case analysis, no additional dis-utilities were applied for other clinical events as the utilities used in the model were based on trial data collected over time, therefore already accounting for the impact of clinical events on quality of life. To test the impact of this assumption, event-specific dis-utilities were applied in the model in a sensitivity analysis (Table 4).
Treatment of uncertainty
Uncertainty in the model was evaluated in several ways. The overall parameter uncertainty of the cost-effectiveness results was assessed using probabilistic sensitivity analysis (PSA). In PSA, the model is run a large amount of times using Monte Carlo simulation. In each simulation, different values of model input variables (eg mortality rates, costs) are drawn based on their specified distribution and parameters. Beta distributions were used to define mortality and other clinical event rates, along with utility variables. Gamma distributions were used to define cost variables. The overall parameter uncertainty is expressed through cost-effectiveness acceptability curves (CEACs) which present the probability that an intervention (TAVI) is cost-effective over a range of willingness-to-pay thresholds (eg $50,000 or $100,000 per QALY gained). Additionally, several one-way sensitivity analyses were undertaken in which the cost-effectiveness of TAVI using SAPIEN 3 compared to SAVR was evaluated when changing the value of a single model variable at a time. This included changing the time horizon of the model, changing the discount rate, using initial hospitalization costs based on all patients regardless of surgical risk, changing the cost of the SAVR device, changing non-device procedure costs (ie hospitalization costs) by ±20%, excluding management costs related to clopidrogel or warfarin costs and applying event-specific dis-utilities to the model.
Results
With incremental cost-effectiveness ratios (ICERs) lower than $30,000 per QALY gained, TAVI with the SAPIEN 3 valve was found to be cost-effective compared to SAVR in both intermediate- and high-risk populations. Table 5 presents the details of the base case analyses for both populations. For patients at an intermediate surgical risk, TAVI using SAPIEN 3 was found to generate 0.48 more QALYs than SAVR and cost an additional $13,473, yielding an ICER of $28,154 per QALY gained. For patients at high surgical risk, TAVI was estimated to generate 0.43 more QALYs than SAVR at an additional cost of $7,362. The resulting ICER for the high-risk population was $17,237 per QALY gained.
 |
Table 5 Base-case cost-effectiveness results |
The CEACs for TAVI using SAPIEN 3 in the intermediate- and high-risk groups are shown in Figure 2. For patients at intermediate surgical risk, the probability of TAVI with SAPIEN 3 being cost-effective was 0.91 and 0.99 at willingness-to-pay thresholds of $50,000 and $100,000 per QALY gained, respectively. These probabilities were 0.93 and 0.99 in the high-risk patient group, respectively. Table 6 presents the cost-effectiveness results under various one-way sensitivity analyses. As shown in this table, the only sensitivity analysis in which the ICER became higher than $50,000 per QALY gained was when the time horizon of the model was reduced to 5 years for the intermediate-risk population. Under this scenario, the ICER for the intermediate-risk group became $65,562 per QALY gained. Changes in SAVR device costs, procedural or non-procedural costs, excluding pharmacological management costs following procedures, or including event-specific dis-utilities had a minimal impact on the cost-effectiveness results.
 |
Table 6 One-way sensitivity analysis: TAVI using SAPIEN 3 versus SAVR |
Discussion
Our results indicate that TAVI using the SAPIEN 3 heart valve is cost-effective compared to SAVR in Canadian patients with symptomatic severe aortic stenosis who are at intermediate or high surgical risk. In Canada, access to TAVI varies considerably with only 7 of the 10 Canadian provinces offering access to TAVI in the fiscal year 2013/2014, which is the most recent data available.27 The same data indicated that the average Canadian rate of TAVI procedures per million was 34 with rates varying from 16 in Manitoba (population of 1.3 million or 4% of the Canadian population) to 61 in British Columbia (population of 4.6 million population or 13% of the Canadian population). Due to health care system structures in Canada, TAVI reimbursement status also varies between Canadian provinces but TAVI, when reimbursed, has generally been restricted to inoperable or high-risk patients.
Two recent Canadian studies in intermediate-risk patients11,12 have reported lifetime ICERs of $46,08311 per QALY gained (TAVI with SAPIEN XT versus surgery) and $76,736 per QALY gained (self-expandable TAVI devices versus surgery).12 In comparison to these two studies, we reported lower ICERs when we evaluated TAVI with the newer SAPIEN 3 valve against surgery. This is in line with recent published US data which indicated that economic outcomes were more favorable with the SAPIEN 3 valve in intermediate-risk patients.10 Consistent with several other cost-effectiveness analyses conducted in Canada13 or the United States,28–31 our results confirm that TAVI is cost-effective in patients at a high surgical risk.
There are several limitations with our current analysis that should be considered when interpreting the results. Firstly, mortality and clinical events used in this evaluation were based on non-randomized evidence, which may bring into question the validity of the relative effects (eg mortality) assumed in the model. To deal with this uncertainty, we conducted probabilistic analyses in which all model parameters were simultaneously varied according to prespecified distributions. Results indicated that there was a probability greater than 0.9 that TAVI with SAPIEN 3 was cost-effective in patients at intermediate or high surgical risk if governments’ willingness-to-pay was $50,000 or $100,000 per QALY gained, two commonly cited thresholds for health care decision-making. Secondly, in common with most economic analyses, outcomes such as mortality had to be extrapolated from data that was shorter than the 15-year time horizon of the model. To evaluate the impact of these extrapolations, we truncated the base case time horizon to 10 and 5 years in sensitivity analyses, which increased the cost-effectiveness ratios to values still deemed cost-effective. The results of several other one-way sensitivity analyses indicated that the results were not sensitive to change in other key variables (eg hospitalization costs, SAVR costs, utility data).
Another limitation of our study is the use of Canadian administrative data to estimate the cost of the initial hospitalization associated with TAVI and SAVR. As such, the hospitalization costs associated with TAVI and SAVR were not adjusted for differences in baseline characteristics (eg TAVI patients older than SAVR patients). We however believe that this is a great strength of our study as it reflects the real-world use of TAVI and SAVR in Canada. This may also explain why the difference in hospitalization costs between TAVI and SAVR was found to be much greater than that found in the US analysis of the PARTNER II trial in which TAVI with SAPIEN 3 was found to dominate SAVR.10 Finally, we compared TAVI using SAPIEN 3 to standard surgical repair and not against other surgical techniques such as minimal invasive aortic valve surgery, fast-track processing of surgical patients or rapid deployment valves such as Intuity or Perceval. However, minimal invasive aortic valve surgical technique and the rapid deployment valves are not widely used yet.32 Another limitation of our study is that the long-term durability of TAVI using SAPIEN 3 is unknown, and while our analyses included re-interventions for TAVI using SAPIEN 3 based on the trial data, the long-term cost of TAVI treatment could have been potentially underestimated. This also applies to SAVR.
Finally, while the STS score has been used in the PARTNER trials to classify patients into intermediate and high surgical risk patients, it was not available in CIHI administrative databases. For this reason, we used the information contained in the administrative database to create a proxy of the log ES to stratify patients at intermediate or high surgical risk, which is less than ideal. In addition, the log ES is out of date and it is currently recommended to use the EuroScore2 to classify AS patients into intermediate or high risk.17 However, the conversion from the clinical laboratory values required for the calculation of the EuroScore2 to administrative data proxies of ICD-10 CA codes (used in Canada) has not yet been derived. It should be acknowledged that any attempt to define patients retrospectively from administrative data is limited as the final decision on therapy is made by the heart team based on several factors not necessarily captured in administrative databases (eg frailty status). To deal with this issue, we conducted a sensitivity analysis in which we varied the hospitalization costs associated with patients at intermediate or high surgical risk by ±20% and also conducted a sensitivity analysis using similar hospitalization costs for the two populations. Results indicated that TAVI with SAPIEN 3 valve was still cost-effective in the two populations under study.
Within these limitations and consistent with previous studies, our results and sensitivity analyses confirmed that TAVI using the SAPIEN 3 valve is cost-effective compared to SAVR in Canadian patients with symptomatic aortic stenosis at intermediate or high surgical risk.
Disclosure
This study was funded by an unrestricted grant from Edwards Lifesciences. Dr Jean-Eric Tarride reports grants from Edwards Lifesciences, during the conduct of the study and personal fees from Edwards Lifesciences, outside the submitted work. Ms Trinh Luong is an employee of Edwards Lifesciences, the manufacturer of SAPIEN 3 valve system. Dr Gordon Goodall is an employee of Edwards Lifesciences, the manufacturer of SAPIEN 3 valve system. Ms Natasha Burke reports grants from Edwards Lifesciences (Canada) Inc, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62(11):1002–1012. doi:10.1016/j.jacc.2013.05.015
2. Bakaeen FG, Rosengart TK, Carabello BA. Aortic stenosis. Ann Intern Med. 2017;166(1):ITC1–ITC16. doi:10.7326/AITC201701030
3. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi:10.1056/NEJMoa1008232
4. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi:10.1056/NEJMoa1103510
5. al. ADPJRMYSCJe. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;371(10):967–968. doi:10.1056/NEJMc1408396
6. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi:10.1056/NEJMoa1514616
7. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321–1331. doi:10.1056/NEJMoa1700456
8. Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J. 2016;37(28):2252–2262. doi:10.1093/eurheartj/ehw112
9. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387(10034):2218–2225. doi:10.1016/S0140-6736(16)30073-3
10. Baron SJ, Wang K, House JA, et al. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at intermediate risk. Circulation. 2019;139(7):877–888. doi:10.1161/CIRCULATIONAHA.118.035236
11. Tam DY, Hughes A, Fremes SE, et al. A cost-utility analysis of transcatheter versus surgical aortic valve replacement for the treatment of aortic stenosis in the population with intermediate surgical risk. J Thorac Cardiovasc Surg. 2018;155(5):1978–1988 e1971. doi:10.1016/j.jtcvs.2017.11.112
12. Tam DY, Hughes A, Wijeysundera HC, Fremes SE. Cost-effectiveness of self-expandable transcatheter aortic valves in intermediate-risk patients. Ann Thorac Surg. 2018;106(3):676–683. doi:10.1016/j.athoracsur.2018.03.069
13. Health Quality Ontario. Transcatheter aortic valve implantation for treatment of aortic valve stenosis: a health technology assessment. Ont Health Technol Assess Ser. 2016;16(19):1–94.
14. Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–2484. doi:10.1016/S0140-6736(15)60308-7
15. Herrmann HC, Thourani VH, Kodali SK, et al. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134(2):130–140. doi:10.1161/CIRCULATIONAHA.116.022797
16. Ontario Ministry of Health and Long Term Care. Schedule of Benefits for Physician Services under the Health Insurance Act. Queen’s Printer for Ontario. Ontario. 2015; c2002.
17. EuroSCORE. Available from: http://www.euroscore.org/. Accessed March 8, 2019.
18. Ontario Ministry of Health and Long Term Care. Drugs funded by Ontario Drug Benefit (ODB) program: e-formulary Toronto: queen’s Printer for Ontario, 2009-2010. Available from: https://www.formulary.health.gov.on.ca/formulary/. Accessed March 8, 2019.
19. Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70(2):252–289. doi:10.1016/j.jacc.2017.03.011
20. Coyle D, Coyle K, Cameron C, et al. Cost-effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Value Health. 2013;16(4):498–506. doi:10.1016/j.jval.2013.01.009
21. Cost of a Standard Hospital Stay · CIHI. Available from: https://yourhealthsystem.cihi.ca/hsp/inbrief?lang=en#!/indicators/015/cost-of-a-standard-hospital-stay/;mapC1;mapLevel2;/.
22. Patient Cost Estimator. Canadian Institute for Health Information (CIHI). Available from: https://www.cihi.ca/en/patient-cost-estimator.
23. Hospital Inpatient Care Case Costs - CMG/Plex - Open Government.
24. Consumer Price Index, monthly, not seasonally adjusted. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1810000401. Accessed March 8, 2019.
25. Bansback N, Tsuchiya A, Brazier J, Anis A. Canadian valuation of EQ-5D health states: preliminary value set and considerations for future valuation studies. PLoS One. 2012;7(2):e31115. doi:10.1371/journal.pone.0031115
26. Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics. 2003;21(3):191–200. doi:10.2165/00019053-200321030-00004
27. Asgar A, Lambert L, Lauck S, et al. Canadian Cardiovascular Society National Quality Report: Transcatheter Aortic Valve Implantation. Canadian Cardiovascular Society. Ottawa. 2016.
28. Fairbairn TA, Meads DM, Hulme C, et al. The cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis at high operative risk. Heart. 2013;99(13):914–920. doi:10.1136/heartjnl-2013-303722
29. Gada H, Kapadia SR, Tuzcu EM, Svensson LG, Marwick TH. Markov model for selection of aortic valve replacement versus transcatheter aortic valve implantation (without replacement) in high-risk patients. Am J Cardiol. 2012;109(9):1326–1333. doi:10.1016/j.amjcard.2011.12.030
30. Reynolds MR, Lei Y, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement with a self-expanding prosthesis versus surgical aortic valve replacement. J Am Coll Cardiol. 2016;67(1):29–38. doi:10.1016/j.jacc.2015.10.046
31. Reynolds MR, Magnuson EA, Lei Y, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results of the PARTNER (Placement of Aortic Transcatheter Valves) trial (Cohort A). J Am Coll Cardiol. 2012;60(25):2683–2692. doi:10.1016/j.jacc.2012.09.018
32. Fatehi Hassanabad A, Vasanthan V, Kent WDT. Minimally invasive surgical aortic valve replacement: an overview of recent advances. Can J Cardiol. 2019;35(2):225–228. doi:10.1016/j.cjca.2018.11.027
33. Mittmann N, Seung SJ, Hill MD, et al. Impact of disability status on ischemic stroke costs in Canada in the first year. Can J Neurol Sci. 2012;39(6):793–800.
34. Blackhouse G, Assasi N, Xie F, et al. Cost-effectiveness of catheter ablation for rhythm control of atrial fibrillation. Int J Vasc Med. 2013;2013:262809.
35. Sullivan PW, Arant TW, Ellis SL, Ulrich H. The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at high risk of stroke in the US. Pharmacoeconomics. 2006;24(10):1021–1033. doi:10.2165/00019053-200624100-00009
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

