Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
A Bidirectional View of Migraine and Diet Relationship
Authors Gazerani P
Received 16 November 2020
Accepted for publication 6 January 2021
Published 11 February 2021 Volume 2021:17 Pages 435—451
DOI https://doi.org/10.2147/NDT.S282565
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Parisa Gazerani1,2
1Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Aalborg, Denmark; 2Pharmacy, Department of Life Sciences and Health, Faculty of Health Sciences, OsloMet, Oslo, Norway
Correspondence: Parisa Gazerani
Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Fredrik Bajers Vej 5B, Aalborg East, 9220, Denmark
Email [email protected]
Abstract: Migraine is a common headache with a large negative impact on health. Several endogenous and exogenous factors can influence the severity and frequency of migraine, for example, lifestyle factors including dietary factors. Consequently, lifestyle modifications and dietary considerations have been reported beneficial to moderate clinical features of migraine. Much effort has been invested in determining the lifestyle factors (eg, stress, exercise, sleep, and diet) that trigger migraine to develop recommendations and guidelines for prevention. Diet has also been investigated with a major focus on the content of the diet and to a lesser extent on the amount, pattern, and quality of diet. Identification of dietary factors in migraine has led to nutritional interventions with a major focus on elimination of triggers, and weight control strategies. Several so-called migraine diets have consequently been proposed, for example, the ketogenic diet. Some theories have considered epigenetic diets or functional food to help in altering components of migraine pathogenesis; however, these theories are less investigated. In contrast, evidence is being accumulated to support that some mechanisms underlying migraine may alter dietary choices, for example type, amount, or patterns. Since a causative relationship is not yet established in migraine-diet relationship as to which comes first, this concept is equally valuable and interesting to investigate. Only limited epidemiological data are available to demonstrate that dietary choices are different among patients with migraine compared with individuals without migraine. Differences are reflected on quality, composition, pattern, and the amount of consumption of dietary components. This view emphasizes a potential bidirectional relationship between migraine and diet rather than a one-way influence of one on the other. This targeted review presents examples from current literature on the effects of diet on migraine features and effects of migraine on dietary choices to draw a perspective for future studies.
Keywords: migraine, headache, diet, food, dietary pattern, lifestyle
Introduction
Interest in headache1 is potentially as old as recorded human history. With all the advancements in understanding and management of headaches over the years, headache in general has remained a major complaint for which patients feel an urge for a medical consult. Costs related to headaches are high and are classified as direct (medical care) and indirect costs (loss of productivity). Therefore, if headaches can be diagnosed correctly and earlier, and if they can be managed properly, the burden to patients and societies will be dramatically reduced.
Based on the latest version of the headache classification, migraine is a form of primary headaches,2 ranked among the most disabling medical conditions.3 Number four of the Trøndelag Health Survey (HUNT4 study) revealed that 18.1% of the studied population had active migraine.4 Migraine is characterized by headache attacks and associated symptoms presented in a multiphasic nature,5 where both peripheral nervous system and central nervous systems are considered involved.5,6 The recurrent nature of migraine and the fact that it can be triggered,7 have provided a key feature to explore internal and external triggers and through those, to study the mechanisms underlying the disorder. This phenomenon has also presented a unique opportunity to modify triggering factors—those that can be modified—to reduce intensity of migraine and how often it occurs. This concept is attractive, as it has been found that lifestyle factors,8 such as diet,9 can trigger migraine, and lifestyle modifications,10 for example diet modifications, and nutraceutical interventions11 have collectively shown beneficial effects in patients with migraine. Considering these options is important, because despite remarkable advancement in understanding of the pathogenesis of migraine and targeting migraine by the novel therapeutic options,6 challenges remain related to sufficient efficacy, and desirable safety, and the fact that nonresponders are present.12 In addition, a number of individuals with migraine are continuously searching for natural and device- or drug-free interventions outside of the typical therapeutic options. In this line, functional medicine approach to manage migraine has been proposed as a potential tool. This approach considers individual's genetic, biochemical, and lifestyle factors to construct plans for personalized treatment. Functional medicine consists of timeline, matrix, and the therapeutic lifestyle factors (for example, sleep, exercise, diet, and stress). Within this framework, functional food can also be defined for migraine. Generally, a food is defined functional if it is satisfactorily demonstrated to affect beneficially one or more target functions in the body, beyond adequate nutritional effects in a way that is relevant either to an improved state of health and well-being and/or reduction of risk of disease.13 Functional food has been tested to identify if it can exert beneficial effects for several diseases, for example for metabolic syndrome.14 This syndrome consists of several metabolic disorders (eg, high levels of fasting glucose and obesity) and enhances the risks of other diseases, for example stroke, diabetes, and cardiovascular diseases.14 Interestingly, migraine has also been recognized as a disorder related to metabolic imbalance, and that highlights a potential for functional food for migraine.15 William Amery in 1982, provided the first evidence that the metabolism is linked to the pathogenesis of migraine.16 Recent studies investigating metabolic alterations in migraine have proposed that a mismatch seems to exist between brain energy sources and the consumption of the sources,17 and have linked this energy deficit to mitochondrial dysfunction in migraine.18 It is hypothesized that energy-reserve deficit alone or combined with an overload of sensory input could activate the trigeminovascular system in the cascade of pathophysiological events in migraine.18 Based on this, a metabolic treatment of migraine has been proposed.17,19
While identification of dietary triggers and dietary interventions for migraine prevention are profound in the literature, the concept of dietary choices, and pattern of diet in migraine patients have been investigated less.20,21 The idea that mechanisms underlying migraine pathogenesis might influence dietary choices is valuable, but has sporadically been discussed.20,21 Epidemiological findings have demonstrated that choice of diet by individuals with migraine is different compared with individuals without migraine. Potential reasons for such difference have been explained by several factors, for example, contribution of neurotransmitters such as serotonin and orexin, hormones, and state of aura.21 A potential bidirectional relationship (Figure 1), where migraine influences food intake, and consumed food affects the manifestations of migraine, needs further investigation. Within this framework, investigation of the gut–brain axis contribution seems highly valuable.21
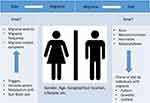 |
Figure 1 A potential bidirectional relationship between migraine and diet. |
In the following sections, some examples from the current literature are presented to highlight what we already know about the effects of diet on migraine and the effects of migraine on dietary choices, and what remains unknown to stimulate further research. Therefore, the purpose of this targeted review is not to provide a comprehensive systematic review of the current literature on the role of diet in migraine; since several excellent reviews are already available (eg,9,22–26). PubMed, Cochrane Library, EMBASE, and Web of Science databases were searched for studies using keywords of diet, migraine, food, and lifestyle with the aim of providing the current overview, and a viewpoint to the potential future directions. The ultimate goal is to form testable scientific hypotheses for future investigation of the bidirectional relationship of migraine and diet.
Studies that have investigated whether and how the consumption of dietary components can influence the manifestations of migraine are abundant. The potential role of dietary triggers, contribution of the immune system, metabolic systems, and the gut–brain axis contribution are among the examples focused on the effect of diet on migraine. The other direction, where migraine might also influence the food intake, has been less investigated. Presence of aura,27–29 some neurotransmitters involved in pathogenesis of migraine (eg, serotonin,30,31 and orexin32), hormones (eg insulin33), and level of adiocytokines34 have been proposed to influence the choice of diet by affected individuals in terms of content, pattern, and amount of food intake.21 Several familiar and unknown factors can potentially influence this bidirectional relationship. These include, but are not limited to, gender, age, and geographical locations.9,24,35–37
How Does Diet Affect Migraine?
Triggers and Trigger Perception in Migraine
A large number and diverse range of factors (eg, dietary factors) with a high degree of heterogeneity have been reported capable of triggering migraine.38 For example, stress has been shown to exacerbate migraine, and having or expecting a migraine can negatively affect stress level of affected individuals. Menstrual migraine is a typical example of the link between hormones and migraine. Sleep and migraine have also been found interrelated where sleep disturbances aggravate migraine. Other environmental factors such as intense light, strong odors and high altitude have also been reported to influence migraine.39 Consequently, long lists of recommendations exist for avoiding potential triggers or coping strategies in order to prevent migraine or subsiding its frequency and severity; hence, enhancing the quality of life in affected patients.40
A meta-analysis of available studies for headache triggers has summarized data from 27,122 participants from 85 articles published between 1958 and 2015, and has provided 420 triggers.41 86% of the included participants in this meta-analysis had the minimum of one trigger for their headaches. Findings from this study highlighted that stress was the most prevalent trigger.41 Heterogeneity, however, was high and intra- and interindividual variations among trigger frequency and potency were also profound.41 Knowledge of migraine triggers can help in improving the management, coping, and care for migraine; but studying migraine triggers is not challenge-free. Using smartphone-based dairy studies that use ecological momentary assessment systems, has presented fatigue, sensory sensitivity, negative affect, specific foods, menstruation, and yawning as the most frequent triggers of migraine.42–44 Correct understanding of trigger perception has been discussed by Turner et al45 to highlight how important are the behavioral changes in response to a headache trigger that is perceived by patients as a precipitating factor. An example is the avoidance of bright light if the individual with migraine perceives it as a migraine trigger. This controlling avoidance behavior may influence the scope of individual activities, and can negatively influence the quality of life.40,46,47 Perhaps that is why coping strategies are prioritized to avoidance strategies, in general.47
Collectively, the current ultimate recommendation for individuals with migraine has pointed to the value of maintaining an appropriate and healthy lifestyle.48 Lifestyle can be defined as the “controlled behavior and activities of a person and many activities, habits, and practices involve risk factors”. The contribution of dietary factors within the lifestyle modification has been recognized; however, proposed beneficial changes in lifestyle, consider a broader spectrum to not only include dietary aspects, but also monitoring of exercise, sleep, and stress.49
Dietary Triggers and Dietary Modifications in Migraine
It has been proposed that modification of lifestyle might prevent migraine, which in turn would decrease the burden to individual patients, and health-related costs.50 However, due to the complexity of migraine, as a multidimensional disorder, and also the complexity of designing studies to test how dietary factors can influence migraine,49 inconsistency exists in the literature, ranging from a limited importance of dietary modification for migraine to some promising effects. Cross-sectional studies have been important in providing an overview of potential triggers;51 however, if the goal is to prove (or falsify) that a causal or a bidirectional relationship exists in the diet–migraine interaction, prospective studies with proper control groups must be designed that are also longitudinal in nature. For example, age of onset is extremely important.51 A migraine patient passes through different phases in an age span, from pediatric to geriatric migraines, for example. Puberty has been shown linked with migraine and migraine that occurs before puberty differs from post-puberty migraine. Several factors such as alteration in lifestyle, habits, and hormonal levels have been proposed to shape this evolution from pre- to post-puberty. A recent study52 has investigated this evolution in a selected pre-pubertal patients who were diagnosed with migraine. Researchers in this study collected medical records, migraine manifestations, and lifestyle-related factors, at baseline and at the two-year follow-up. Nineteen patients (migraine with aura: 27.5%) were recruited. The results of this study demonstrated that migraine accompanying symptoms changed with a significantly higher prevalence of dizziness, vertigo, mood changes, confusion, and allodynia.52 Prodromal symptoms became more prevalent, where sleep disturbances and schedule changes showed a significant increase as migraine triggers. Interestingly, at baseline, food was triggered at 11% of cases, but after two years, it went down to zero. Another study on the participants aged above 16, has also identified the appearance of new triggers over time. For example, new factors, including pain in neck, consumption of alcohol, hormonal changes, and smoking were notified.53 These studies provide valuable information that migraine triggers show changes during puberty, and new triggers can appear together with changes in habits along with physical and lifestyle changes, which collectively highlight attention to a potential dynamic pathological process that deserves further investigation. These studies also present a valuable point that besides studying dietary factors other daily lifestyle features, for example how a patient sleeps, makes a work–rest balance, and deals with stressful situations are important to observe and note, because these factors are often interrelated and can influence each other directly or through indirect interactions. Comorbid conditions, such as other neurological, psychological, or cardiovascular disorders are also important and influential, because patients, influenced by those conditions, might follow a special lifestyle, including certain diets.1 For example, those who are diabetic or have a heart disease may follow a vegan diet for its beneficial effects,54,55 and some migraine patients might be on a vegan diet for comorbidy or other reasons.
A systematic review from 202022 has summarized the findings from 43 studies that have investigated and reported dietary patterns (11 studies), triggers (20 studies), and dietary interventions (12 studies) in patients with migraine.22 Level of evidence was determined as low level, because the authors identified that >50% of the studies were cross-sectional or patient surveys. Caffeine and alcohol were found as major triggers that could increase migraine frequency.22 Several dietary interventions were also reviewed, for example, elimination diets, low-fat diet, and ketogenic diet that presented promising results in managing migraine.22 However, this review did not present a choice or a favorable, so-called migraine diet, due to lack of qualified and sufficient information.
Elimination diets can be based on a diary for identification of triggers, or based on tests for the IgG-positive food, both strategies to limit those triggers. When participants were tested for antibodies against 266 foods and individually eliminated those foods that they had positive tests for, a reduction of 29% in migraine days was found.56 This study was, however, a small cross-sectional study, with some limitations. Another study, which was designed as a randomized controlled trial, eliminated those foods from diets of migraine patients who participated and were positive for certain food-related antibodies. When headache days were determined after four weeks on the elimination diet, a 19% reduction was found.57
Dietary interventions have mostly been investigated in a small population with no proper control group, hence results are heterogeneous and a sharp conclusion cannot be made. For example, a diet high in carbohydrate and low in tryptophan was tested in a group of seven patients and showed beneficial to subside headaches. The authors proposed that the positive effect has been apparently due to a mixture of lower intake of food that could trigger headache and also elevated levels of serotonin following the tested diet.58
Dietary lipids were investigated afterwards, because it was proposed that a diet high in lipids could cause headache following a potential lowering of serotonin levels in plasma that might be a result of higher platelet aggregation.59 A diet with a very low level of lipids (~20 g per day), therefore, was proposed to prevent headaches.60 A randomized, crossover trial reported in 201561 that low lipid compared with moderate lipid dietary intake could subside occurrence of migraine and headache intensity. It has also been reported that the dietary approaches to stop hypertension (DASH) diet could diminish the intensity of headache and duration in migraine.62 This particularly points to the importance of migraine comorbidities, and how dietary factors can influence an overall well-being of the affected patient.
Supplementation by a diverse range of vitamins and minerals has been reported beneficial for migraine. For example, based on a review from 2018, vitamin D, vitamin B2, vitamin B12, magnesium, carnitine, and niacin have reduced frequency of magnesium, carnitine, and niacin have reduced frequency of migraines.63
Even though beneficial effects of these dietary interventions have been reported in the literature,22 one must consider that individual patients may require special needs that importantly points towards the concept of precision medicine in migraine.64 Including larger cohorts of patients and considering follow-ups of longer duration could help in properly examining the effect of dietary interventions, a point to be considered in the future investigations. In this line, patient adherence and age influence on diet choices and dietary patterns emphasize the value of long-term assessments. However, plan, design, and conduct of long-term studies are difficult and several intractable factors need to be considered and integrated into the assessments. At present, comparisons between studies remain difficult because age, gender, cultural, and religious variations among different studied populations have largely been ignored. Gender of affected individuals is an important factor to consider,51 because changes in hormonal concentrations, for example plasma estrogen concentrations, have shown an association with migraine.65 Alternatively, dietary intakes that can alter estrogen activity to a lower level have been shown beneficial for premenstrual symptoms.66 Therefore, low fat, high fiber, or vegan diets, might help some patients, for example those who have menstrual migraines. In fact, a study67 has tested this hypothesis, by investigating the effects of a four-week low-fat vegan diet in migraine. Overall, headache severity, headache days and frequency subsided, but this study has some limitations in design preventing drawing a sharp conclusion.67 Besides linking beneficial effects of a vegan diet to a low fat content, and lowering estrogen activity, several other mechanisms have been proposed, for example antioxidant and anti-inflammatory properties of plant-based food. Since an involvement of neurogenic inflammation in migraine68 has been suggested, this might be an explanation. In addition, dairy products (eg, cheese) and meat49 are not present in a vegan diet and these components have often been reported as migraine triggers in the literature.69,70 Therefore, absence of these components in a vegan diet might exert an anti-inflammatory effect against migraine.
Obesity–Migraine Linkage and Weight Control in Migraine
Weight loss has been reported beneficial in migraine,71,72 although open questions remain in the field due to design and studied populations in the current literate. A proof of concept study in 2015 presented that weight loss could result in symptom improvement.73 Based on a pilot study published in 2019,11 enhancing the quality of diet and maintaining a healthy weight, could improve some clinical features of migraine. In this open, and nonrandomized study, women with migraine received an individualized diet plan, which was based on a professional nutritional diagnosis. This study was first to provide evidence that diet quality and maintaining a healthy weight are important,11 not the weight loss per se. This means that for underweight patients a weight gain might be the successful strategy, while for overweight patients, a weight reduction strategy would provide beneficial effects on migraine.11
Bond et al74 designed a study to test if two different strategies for weight loss would be comparable or different. Migraine patients who were overweight or obese women (a population considered to be most affected by obesity‐related migraine risks)35,75,76 were included and divided into two groups. One group received a behavioral weight loss (BWL) that included both exercise and diet, and the other group received educational instructions on migraine. Findings from this study showed that both groups benefited from a reduction in headaches following the two strategies and there was no significant difference between the groups. This study presented that independent of the type of strategy; strategies for weight loss might be beneficial for this special population.
According to a systematic review and meta-analysis77 that has summarized and compared two strategies for weight loss, it was revealed that independent of technique, weight loss could reduce headache severity, frequency, duration, and associated disabilities. Therefore, weight loss was highlighted as the critical factor, not the amount of weight reduction, or the strategies that were used to achieve the loss.77 In fact, the obesity and migraine link has been a matter of investigation for a while. The fundamental questions are, do people with migraine gain weight because of migraine related disability? Or does obesity lead to greater migraine frequency? In other words, which comes first, obesity or migraine. Results are mixed in the literature. Winter et al in 201278 found that among 19,162 middle-aged women, those with migraine had a significantly higher risk to shift towards being overweight or obese. The risk was not different for women with or without aura.78 Age plays a role in obesity–migraine interactions,79 because age affects the body mass index (BMI), distribution of body fat, hormones, and prevalence of migraine. Reported in 2020, the HUNT3 (the third population-based Nord-Trøndelag Health Study)80 showed that a greater association exists between migraine and obesity in younger adults, ie, those >50 years old, still within the reproductive age. Therefore, one must consider that in the study by Winter et al,78 where middle-aged women were included, other risk factors might have played a role.
A meta-analysis81 of 12 studies, including data from 288,981, demonstrated that body composition is a critical factor. When pooled data were adjusted for age and sex in this analysis, an increase risk of migraine (27%) was identified in obese vs normal weight and was not lost even after multivariate adjustments. The risk was shown slightly elevated (13%) in underweight vs normal weight and again it was not changed even after application of multiple adjustments. Therefore, it seems based on these results, that obesity and being underweight could enhance risk of migraine.81 An increased risk of migraines in underweight and obese women vs normal weight was presented in 2015 by Ornello et al.82 However, pre-obese subjects did not show any increasing risk.82
Multiple underlying mechanisms for the impact of obesity on migraine have been proposed, one of which is a neurometabolic impact.18 This has been based on reports in the literature that metabolic factors can trigger migraine, for example, fasting/hypoglycemia, dehydration, stress, alcohol, and lack of sleep. These factors have been found linked to reduced brain energy levels in migraine patients. It has been proposed that these triggers could reduce mitochondrial function, ATP generation, cellular glucose transport, and lipid oxidation, promote neuroinflammation (neuronal and glial signaling modulation), and astrocytic signaling.18,19 These mechanisms are also linked to increased cortical excitability that has been proposed in migraine pathophysiology.83 The review by Gross et al18 in 2019, summarized the available literature on the metabolic changes in migraine and how those changes can contribute in pathophysiology and being potential targets for treatments. One important feature in this context is that nutritional intervention to improve nutrient metabolism, neuroinflammation, and oxidative stress, can eventually improve migraine.18 This has shed light on the concept of obesity and migraine. Observations have provided evidence that the hypothalamus which is the first station for detecting of changes in peripheral energy status, is involved in migraine pathogenesis.84 Interestingly, it has been found that hypothalamic astrocytes have distinct responses to nutrients, ie fatty acid and glucose metabolism coupling.85 In addition, it has been found that different brain cells utilize, store, and modify their response to lipids. L-carnitine, which transports fatty acids into the mitochondria, where those are oxidized to produce ATP, has shown efficacy in blunting migraine.86 In contrast, saturated high-fat diets leading to obesity, promote metabolic dysfunction, depressive like behavior, and neuroinflammation.87 This has led to applying a strategy in which targeting obesity could suppress neuroinflammation and consequently block the depressive symptoms. Interestingly, increased mood disorders have been seen in migraine patients, so these basic research findings are clarifying some underlying mechanisms that might share commonalities in obesity, migraine, and mental health.88
The concept that migraine might be a response to low brain energy level or uncompensated oxidative stress,89 has brought the ketogenic diet back into attention.90 This diet acts in a similar way to fasting, where ketone bodies are elevated and can be used as an alternative source of energy to correct abnormalities in glucose metabolism reported in migraine. Some reports, including a proof of concept study,73 have demonstrated beneficial effects of a ketogenic diet to reduce migraine frequency. Recently, an alternative method has been considered to apply exogenous ketogenic substances.91 This means to provoke nutritional ketosis with ketogenic substances, for example, beta-hydroxybutyrate (βHB) salts.91 A recent review90 summarized the potential mechanisms underlying the effect of ketone bodies and presented those as signaling molecules that can interfere with pathways involved in migraine pathophysiology.90 For example, ketogenic substances can reverse mitochondrial dysfunction, subside oxidative stress, reduce cerebral excitability, or lower the inflammation.90 Even though an extensive amount of work has been done in animals, clinical research is lacking to validate the findings as if these protective effects of ketone bodies (KBs) would also be present in patients with migraine. Supplementation with βHB without a strict dietary change is under investigation91 and could help provide evidence and address those open questions.
Diet-induced obesity has been shown to reduce brain fatty acid uptake.92,93 This has opened up a concept that obesity could enhance deficits in brain energy reserves and metabolism that characterize migraine. Within this concept, omega-3 fatty acid supplementation has shown antidepressive action and reduced migraine frequency.94 Fish oil supplementation in obese mice95 has shown reduction in metabolic and anxiodepressive effects of diet-induced obesity and related alterations in the composition of brain lipid. Further investigation is required in humans, as mood, food, and obesity have been found interrelated in a complex interaction.96 In addition, it is still not known whether a migraine–obesity association is different in females and males, in different ages, and in different subtypes of migraine, considering mood disorders and emotional behaviors in humans.
As the evidence continues to accumulate, it is suggested that physicians recommend weight loss to their patients who have comorbid obesity. This is because weight loss has proven to improve sleep, mood, and other factors that increase susceptibility for having more frequent or severe migraine attacks. Lifestyle changes overlap with migraine and can be beneficial in migraine management, in particular when migraine is comorbid with other conditions, such as depression. There are lifestyle modification approaches for obesity. For example, according to Wadden et al,97 diet, exercise, and behavioral therapy were major determinants of lifestyle modification, where a reduced-calorie diet and a high level physical activity could yield a long-term weight loss.97 Based on a recent review,26 diets that promote weight loss, such as the ketogenic diet, and low-calorie diets, could be considered beneficial for those headache patients who are obese. In addition, lowering intake of omega-6 and intake of higher amount of omega-3 in this group can be advantageous. However, another review9 has emphasized that the net outcome depends on several factors, for example, age, gender, genetic predisposition, and environmental factors. Therefore, in order to provide evidence-based dietary recommendations for migraine, we need to consider these influential factors in study designs. In addition, the more we know about the mechanisms leading to migraine, the better we can investigate different factors, including dietary factors, which can interfere with those mechanisms. Future research is needed to provide evidence of whether diet can be a disease-modifying agent for migraine, and how. Considering the big picture, this would also enable personalized recommendations that - are in line with biopsychosocial considerations in targeting migraine.
In addition, one must consider that if comorbidities exist with migraine, dietary modification might be beneficial in controlling the condition. For example, several studies have highlighted a solid link between migraine and gastrointestinal diseases, in particular, irritable bowel syndrome (IBS). For review see Camara-Lemarroy et al.98
Gut–Brain Axis, Microbiome, and Migraine
The “gut–brain axis” is a term to describe a potential two-way relationship between the gut and the brain. The gut–brain axis might potentially explain the existing link between IBS and migraine.98 Evidence is accumulating on the role of gut–brain axis in several neurological disorders, and migraine is not an exemption, where this has been reviewed in a recent review.99 However, we still do not know how the gut and the brain may interact in migraine.99 Several mechanisms have been proposed,100 for example, composition of gut microbiota, proinflammatory substances such as interleukins, neuropeptides (eg, calcitonin gene-related peptide; CGRP), hormones, and dietary components.101
In a recent metagenome-wide association study (MWAS),102 fecal samples of elderly women with migraine have been compared with matched controls to determine if gut microbiota is associated with migraine. Results showed that patients and controls are different in terms of diversity of species in the gut. Clostridium species (an unhealthy composition) were significantly higher in the migraine group. However, a healthy composition (eg, Faecalibacterium prausnitzii, Bifidobacterium adolescentis, and Methanobrevibacter smithii) were profound in controls. Patients also presented a diminished metabolic function of the gut compared with the controls.102 These findings may pave the way toward diagnosis, prognosis, and response to treatment strategies, or point to a novel therapeutic target. Based on the results,102 and to maintain healthy composition of the gut microbiota, proper probiotics have been suggested to correct dysbiosis in migraine patients. The concept of using probiotics for maintaining well-being is not new,103 however, identification of the role of probiotics in minimizing neuroinflammation, a mechanism proposed for migraine,104 has attracted attention toward the use of probiotics for alleviating migraine attacks.105,106 In patients with episodic and chronic migraine, a multispecies probiotic supplement has been investigated to identify a potential beneficial effect and profile of inflammatory markers.106 Findings revealed that probiotic supplementation could reduce the frequency and severity of migraine attacks. In addition, patients had a lower number of migraine days in the month and consumed a lower number of drugs to stop migraine headaches.106 According to the findings by Sensenig et al, mineral and vitamins added into a probiotic regimen for 12 weeks could result in a remarkable improvement in headache in 60% of migraine patients. Improvement in quality of life was reported by 80% of patients.107
Probiotic interventions as a prophylactic way to treat migraine have been summarized in a recent systematic review.108 Out of 68 screened studies, only two studies were analyzed, one with negative 109 and one with positive outcome106 in diminishing migraine frequency and intensity. The authors of this review108 have recommended points for inclusion and exclusion for the enrolment of patients, considerations for study design that can recruit standard and comparable methods, and proper control groups, within sufficient time.108 Microbiome analysis, pre- and postintervention, has also been encouraged.108
Another potential explanation for the existing link between gastrointestinal disorders and migraine is the gut permeability,110 where the leaking of lipopolysaccharides from the lumen into the blood can trigger a proinflammatory response,111 which is known to play a role in migraine pathogenesis.112 In a group of migraine patients diagnosed with comorbid IBS, probiotics combined with an elimination diet were tested.113 Sixty patients were randomized into three groups to receive the elimination diet, probiotics, or diet plus probiotics.113 The study results demonstrated that the combination method was superior for improving migraine comorbid with IBS.113
In addition to gut composition, which was found different in migraine patients, collected samples from the oral cavity of patients with migraine have demonstrated different composition from controls.114,115 Significantly higher nitrate, nitrite, and nitric oxide reductase genes were found in oral cavity samples of migraine patients. Interestingly, nitrates and food additives are reported among headache triggers, and nitric oxide pathway has been linked to migraine.116,117 Therefore, bacterial composition can be investigated in oral cavity and fecal samples in migraine and composition might reveal differences from controls.114
Diet, CGRP, and Migraine
Identification of the CGRP role in migraine, has led to the development of new targets118 such as monoclonal antibodies that target CGRP itself, or its receptor, and also new oral gepants, antagonists of CGRP receptor.119 Evidence is limited as if dietary components could interfere with CGRP in migraine. Cady and Durham treated rats with cocoa-enriched diets for 14 days and investigated the expression of CGRP in the trigeminal ganglion cells, where they reported a significant decrease in the expression.120 In cell models, CGRP secretion has also been diminished after treating cells with petasin, which is the active component of butterbur, grape seed, and ginger extract.121,122
In relation to CGRP, a new study123 has demonstrated that when migraine patients with episodic migraine were supplemented with vitamin D, they had lower headache days and disability assessed on the migraine-related disability score (MIDAS) showed a significant improvement after 12 weeks.123 Researchers in this study analyzed the serum levels of CGRP and presented that in the group on vitamin D supplementation, CGRP level was significantly lower.123 Based on the findings and correlational analysis, the authors have proposed that vitamin D might exert some of its effect through lowering of the CGRP levels.123 A larger study with a longer duration together with supportive basic research studies to look into underlying mechanisms of vitamin D in lowering CGRP and exertion of antinociceptive effect through this path, have been suggested.123
Considering beneficial effects of targeting CGRP with recent compounds,118,119 this line of investigation remains open to identify how dietary components or patterns might interact with expression and function of CGRP to interact with migraine manifestations.
Dietary Consistency and Migraine
Neurologists often encourage their patients with migraine to follow a consistent lifestyle. This is based on the observation that sudden changes in any lifestyle component may provoke migraine attacks. This includes several components, such as exercise, sleep, work–rest cycles, diet, etc. However, evidence is still limited. In addition, the pattern of diet or habits of dietary choices might be equally as important as content of the diets. A cross-sectional study in 2015124 that used logistic regression, found that migraine is associated with low intake of food, regardless of the type of food.
A review125 on dietary consistency has presented the topic from three different views to migraine. The authors have proposed migraine as an illness, a disease, and a state of inflammation.125 Within this proposed framework, the authors looked at the relationship between diet and migraine as a function of changes in these three.125 Other researchers have considered migraine a brain disorder of maladaptive response and have described a feedforward allostatic cascade model that can lead to migraine.126 In this model specific stressors such anxiety, noise, food, odors, and bright light can be tested. Each of these factors can contribute to the allostatic load with a different magnitude, and factors can be summed over time. Therefore, the authors have proposed that modification of these effectors or stressors can help to intervene with the skewed allostatic load in migraine.126 Independent of the viewpoint to migraine, maintaining consistency in daily living is not easy and most likely requires education, monitoring, and support, and scientifically driven patterns.125
Does Migraine Affect Diet, and How?
Studies are vast in the literature to examine dietary triggers for migraine and to lesser to examine dietary intervention. However, the question remains open as to whether certain dietary intake patterns are specific to migraine and whether migraine pathogenesis would influence dietary choices and patterns. In this line, it is important to identify if the subtypes of migraine can have an influence on the choices. For example, if the state of aura would lead patients with migraine to select a specific dietary component or patterns, while those choices might be different from those patients who do not have aura, and in comparison with migraine-free individuals. To address this side of the diet-migraine relationship, studies with proper control groups, such as nonheadache and nonmigraine control groups, and including subtypes of migraine (episodic, chronic, with and without aura) would allow for a proper evaluation. However, the evidence is very limited. Pattern of food intake has been investigated in one study,124 where a large population of middle-aged women was included. This study124 was designed based on a hypothesis that migraine patients and healthy individuals are different when it comes to food intake and food avoidance behavior, and that subtypes of migraine (eg, with and without aura) may influence these behaviors even further. This study124 demonstrated that a migraine-specific pattern of food intake existed that was different from healthy individuals. The only exception was alcohol consumption. In addition, and based on the presence or absence of aura in migraine subtypes, the choice of certain food items was influenced. Those items were, for example, chocolate, processed meats, dairy products, and wine.124 Interestingly, lower intake of dietary compounds known as migraine triggers was not evident. This led to an assumption that those food items might have been avoided by patients within a particular subtype of migraine.124 Further studies, however, are required to investigate this arm of migraine–diet relationship. Epidemiological findings have demonstrated that choice of diet by individuals with migraine is different from individuals without migraine and the difference reflects on several nutritional metrics,21 for instance, diet quality,127 diet composition,128 dietary schedule,50 and amount of consumption in a wide range of different foods.23,124,129 We still do not know if mechanisms underlying migraine pathogenesis might influence dietary intake.20,21 Future studies are warranted to identify the patterns and potential underlying mechanisms and to examine if migraine type, migraine frequency, and food intake are interrelated. Besides, longitudinal studies are preferred to cross-sectional studies.
Migraine pain and related disturbances may influence individuals with migraine to select a convenient, simple, or easy choice in diet, which might differ from those without migraine that have a tendency for a more complex dietary pattern. The choice can reflect on the amount, quality, timing, and patterns of dietary intake. This might be due to the fact that the hypothalamus has been found activated in the premonitory phase of migraine, the time that food cravings often occur.28,130 Food cravings, for instance for chocolate, have been reported to present and have accounted for triggering migraine attacks while this might be a part of the onset.1 Interestingly, chocolate has been a matter of investigation as one of the migraine triggers.131 A recent systematic review132 has looked into 25 studies that evaluated if chocolate acts as a trigger in migraine, where 23 studies reported that chocolate could trigger migraine. There were also three provocative studies133–135 that tested the triggering effect of chocolate compared with placebo, and neither of those could identify a significant outcome. Therefore, based on these findings, the authors of the systematic review concluded that evidence is still lacking to draw any recommendation for migraine patients about eating or avoiding chocolate.132
Neurotransmitter, hormone, and adipocytokine levels in migraine patients are different compared with controls that might also influence the desire for food, or food intake or even the metabolic control of the hypothalamus18,136 in affected patients. For example, orexin A, was elevated in headache phase,32 while serotonin levels were lower during the interictal phase.30,31 Higher insulin resistance and elevated adipocytokines such as leptin are also reported in migraine patients compared with controls.33,34
The choice of mealtime by migraine patients might also affect the meal intake and its properties. There is a gap here for understanding how migraine history would influence a preferred mealtime in an attempt to manage migraines proactively. Mealtime can influence the content of meal depending on the time, and hence plays a role in the bidirectional loop of migraine-diet. In fact, a study from 2016137 has looked into the pattern of regular lifestyle behavior for three elements of sleep, mealtime, and daily exercise in patients with episodic and chronic migraine. This is the first study of the combined three variables compared with previous studies138–140 that considered each domain separately. Findings from this study137 demonstrated that all three elements (ie, regular mealtime, regular sleep, and daily exercise) were lower in frequency among migraine patients with chronic migraine compared with episodic migraine. Interestingly, regular mealtime was found as the element that was adopted the best by both groups of migraine patients.137 The authors, therefore, proposed that self-regulated behaviors, such as regular mealtimes, would be beneficial for the affected patients to control their migraine.137 It is interesting to investigate whether genetic or epigenetic factors64 can influence the choice of mealtime by patients with migraine and if this differs between episodic and chronic migraine.
A small number of patients have been seen anecdotally to respond to the paleo diet or variations of this diet. The rationale follows a theory that modern era diseases, for example, diabetes, heart disease, and obesity were absent in the Paleolithic era. Therefore, a clear diet could also help prevent migraine. This diet is known for weight loss, and it is free from refined and processed food, additives and preservatives.
The gut–brain axis that is a bidirectional path, might also affect dietary choices here. Interestingly, the gut–brain axis has been discussed in terms of psychological aspects,141 named as gut–brain psychology, which brings mind to the equation of the brain and gut relationship. Based on this synchronism of gut, brain, and mind, it has been proposed that the gut microbiota could affect normal mental processes and under pathological mental and neurological disorders.141 Whether this can influence choice of diet in migraine, or when migraine is co-existent with other psychological conditions, eg, stress and anxiety, needs further investigation. This also remains to be tested as if other factors, eg, gender of migraine patients can affect this (by, eg, hormones or psychology-related factors). Figure 2 is an overview of the gut–brain axis and potential players in the bidirectional relationship of migraine and diet.
 |
Figure 2 A bidirectional relationship of the gut and brain, and different factors that can potentially influence migraine–diet bidirectional relationship within this system. Green arrows are toward improvement of migraine headache, while red arrows reflect on negative impact. For a comprehensive review on the gut–brain axis and migraine headache, please see Arzani et al.99 |
Taken together, a potential bidirectional relationship, where migraine influences food intake, and consumed food affects the manifestations of migraine, needs further investigation. The question, therefore, remains open as to whether migraine can affect dietary choices and to what extent, and how dietary choices can influence migraine. In a broader spectrum, the allostatic model in migraine126 could potentially help studying the influence of migraine on food intake and the influence of dietary intake on migraine. Table 1 provides an overview of the main points mentioned earlier for the diet–migraine relationship and considerations for future studies.
 |
Table 1 A Summary of Main Elements in Bidirectional Aspects of Diet–Migraine and Migraine–Diet Relationship |
Concluding Remarks
Diet as a potential trigger for migraine has been discussed for some time. Identification of potential dietary triggers for migraine125 has mainly emerged via keeping dairies, avoidance behavior, or elimination diets to help managing migraine.142,143 Some triggers appear common among the migraine population, while others appear to be unique to individuals. Therefore, identification of personal food triggers in each individual seems valuable to assist with a better way of coping with migraine. No particular migraine diet exists yet to lean on a strong evidence, and hence the investigation of dietary patterns is needed to confirm efficacy before recommending for migraine prevention. Types of evidence, including level of effect are, therefore, expected from these interventions. For each, one must consider the burden of various diets for patients and if any potential side effects or safety issues may occur.20
Comorbidities are also important to consider, such as IBS and in this regard, studying the role of the gut–brain axis is encouraged. Migraine has been also associated with cardiovascular and psychological disorders. Therefore, studying dietary interventions that can be beneficial for comorbid conditions are valuable. Dietary recommendations for migraine may aid in immediate control, slow progression, or prevention of diet-related comorbidities (eg, obesity, diabetes, and cardiovascular diseases). These recommendations are often included in a broader lifestyle modification, including sleep hygiene, stress management, regular exercise, or smoking cessation. A focus on maintenance of a consistent healthy lifestyle, in addition to nonpharmacological and pharmacological management of migraines seems to be the key for most of migraine patients.20 Implementation of any lifestyle changes, including dietary factors, needs a careful evaluation and a clear communication to help both clinicians and patients to achieve expected and reasonable goals. Education, monitoring, and support are essential elements in particular in long-term interventions and follow-ups.20 Effect of migraine or its evolution over age and among the genders for dietary choices, and dietary pattern is not known.51 Pattern, quality, and amount of food can also be influenced by geographical locations, cultural, and religious factors. These factors must be considered and reported in future studies of any potential bidirectional relationship between migraine and diet.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Rizzoli P, Mullally WJ. Headache. Am J Med. 2018;131(1):17–24. doi:10.1016/j.amjmed.2017.09.005
2. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
3. Collaborators GBDH. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–976.
4. Hagen K, Asberg AN, Uhlig BL, et al. The epidemiology of headache disorders: a face-to-face interview of participants in HUNT4. J Headache Pain. 2018;19(1):25.
5. Dodick DW, Phase-by-Phase A. Review of migraine pathophysiology. Headache. 2018;58(Suppl 1):4–16.
6. Ashina M. Migraine. N Engl J Med. 2020;383:1866–1876.
7. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619–6629.
8. Hagen K, Asberg AN, Stovner L, et al. Lifestyle factors and risk of migraine and tension-type headache. Follow-up data from the Nord-Trondelag Health Surveys 1995-1997 and 2006-2008. Cephalalgia. 2018;38(13):1919–1926.
9. Gazerani P. Migraine and diet. Nutrients. 2020;12:6.
10. Robblee J, Starling AJ. SEEDS for success: lifestyle management in migraine. Cleve Clin J Med. 2019;86(11):741–749. doi:10.3949/ccjm.86a.19009
11. Costa ABP, Rodrigues A, Martins LB, et al. Nutritional intervention may improve migraine severity: a pilot study. Arq Neuropsiquiatr. 2019;77(10):723–730.
12. Schytz HW, Hargreaves R, Ashina M. Challenges in developing drugs for primary headaches. Prog Neurobiol. 2017;152:70–88.
13. Scientific concepts of functional foods in Europe. Consensus document. Br J Nutr. 1999;81(Suppl 1):S1–27.
14. Khan MI, Anjum FM, Sohaib M, Sameen A. Tackling metabolic syndrome by functional foods. Rev Endocr Metab Disord. 2013;14(3):287–297.
15. Plasek B, Lakner Z, Kasza G, Temesi A. Consumer evaluation of the role of functional food products in disease prevention and the characteristics of target groups. Nutrients. 2019;12:1.
16. Amery WK. Brain hypoxia: the turning-point in the genesis of the migraine attack? Cephalalgia. 1982;2(2):83–109.
17. Hassan SA, Farooque U, Choudhry AS, Pillai B, Sheikh FN. Therapeutic implications of altered energy metabolism in migraine: a state-of-the-art review. Cureus. 2020;12(6):e8571.
18. Gross EC, Lisicki M, Fischer D, Sandor PS, Schoenen J. The metabolic face of migraine - from pathophysiology to treatment. Nat Rev Neurol. 2019;15(11):627–643.
19. Lisicki M, Schoenen J. Metabolic treatments of migraine. Expert Rev Neurother. 2020;20(3):295–302.
20. Slavin M, Ailani J, Clinical A. Approach to addressing diet with migraine patients. Curr Neurol Neurosci Rep. 2017;17(2):17.
21. Slavin M, Li HA, Frankenfeld C, Cheskin LJ. What is needed for evidence-based dietary recommendations for migraine: a call to action for nutrition and microbiome research. Headache. 2019;59(9):1566–1581.
22. Hindiyeh NA, Zhang N, Farrar M, Banerjee P, Lombard L, Aurora SK. The role of diet and nutrition in migraine triggers and treatment: a systematic literature review. Headache. 2020.
23. Zaeem Z, Zhou L, Dilli E. Headaches: a review of the role of dietary factors. Curr Neurol Neurosci Rep. 2016;16(11):101.
24. Yamanaka G, Morichi S, Suzuki S, et al. A review on the triggers of pediatric migraine with the aim of improving headache education. J Clin Med. 2020;9:11.
25. Geiselman JF. The clinical use of igg food sensitivity testing with migraine headache patients: a literature review. Curr Pain Headache Rep. 2019;23(11):79.
26. Razeghi Jahromi S, Ghorbani Z, Martelletti P, Lampl C, Togha M. School of advanced studies of the european headache F. Association of diet and headache. J Headache Pain. 2019;20(1):106.
27. Neut D, Fily A, Cuvellier JC, Vallee L. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13(1):61–65.
28. Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47(10):1418–1426.
29. Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2014;137(Pt 1):232–241.
30. Ren C, Liu J, Zhou J, et al. Low levels of serum serotonin and amino acids identified in migraine patients. Biochem Biophys Res Commun. 2018;496(2):267–273.
31. Rossi C, Pini LA, Cupini ML, Calabresi P, Sarchielli P. Endocannabinoids in platelets of chronic migraine patients and medication-overuse headache patients: relation with serotonin levels. Eur J Clin Pharmacol. 2008;64(1):1–8.
32. Sarchielli P, Rainero I, Coppola F, et al. Involvement of corticotrophin-releasing factor and orexin-A in chronic migraine and medication-overuse headache: findings from cerebrospinal fluid. Cephalalgia. 2008;28(7):714–722.
33. Rainero I, Govone F, Gai A, Vacca A, Rubino E. Is migraine primarily a metaboloendocrine disorder? Curr Pain Headache Rep. 2018;22(5):36.
34. Dominguez C, Vieites-Prado A, Perez-Mato M, et al. Role of adipocytokines in the pathophysiology of migraine: a cross-sectional study. Cephalalgia. 2018;38(5):1005–1006.
35. Peterlin BL, Rosso AL, Williams MA, et al. Episodic migraine and obesity and the influence of age, race, and sex. Neurology. 2013;81(15):1314–1321.
36. Tai MS, Yap JF, Goh CB. Dietary trigger factors of migraine and tension-type headache in a South East Asian country. J Pain Res. 2018;11:1255–1261.
37. Millichap JG, Yee MM. The diet factor in pediatric and adolescent migraine. Pediatr Neurol. 2003;28(1):9–15.
38. Camboim Rockett F, Castro K, Rossoni de Oliveira V, da Silveira Perla A, Fagundes Chaves ML, Schweigert Perry ID. Perceived migraine triggers: do dietary factors play a role? Nutr Hosp. 2012;27(2):483–489.
39. Wober C, Holzhammer J, Zeitlhofer J, Wessely P, Wober-Bingol C. Trigger factors of migraine and tension-type headache: experience and knowledge of the patients. J Headache Pain. 2006;7(4):188–195.
40. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14(3):221–227.
41. Pellegrino ABW, Davis-Martin RE, Houle TT, Turner DP, Smitherman TA. Perceived triggers of primary headache disorders: a meta-analysis. Cephalalgia. 2018;38(6):1188–1198.
42. Houtveen JH, Sorbi MJ. Prodromal functioning of migraine patients relative to their interictal state–an ecological momentary assessment study. PLoS One. 2013;8(8):e72827.
43. Giffin NJ, Ruggiero L, Lipton RB, et al. Premonitory symptoms in migraine: an electronic diary study. Neurology. 2003;60(6):935–940.
44. Quintela E, Castillo J, Munoz P, Pascual J. Premonitory and resolution symptoms in migraine: a prospective study in 100 unselected patients. Cephalalgia. 2006;26(9):1051–1060.
45. Turner DP, Jchtay I, Lebowitz AD, Leffert LR, Houle TT. Perceived migraine triggers understanding trigger perception can improve management. Pract Neurol. 2018;37–41.
46. Martin PR. Managing headache triggers: think ‘coping’ not ‘avoidance’. Cephalalgia. 2010;30(5):634–637.
47. Martin PR, MacLeod C. Behavioral management of headache triggers: avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29(6):483–495.
48. Mahmoudzadeh Zarandi F, Raiesifar A, Ebadi A. The effect of orem’s self-care model on quality of life in patients with migraine: a randomized clinical trial. Acta Med Iran. 2016;54(3):159–164.
49. Sun-Edelstein C, Mauskop A. Foods and supplements in the management of migraine headaches. Clin J Pain. 2009;25(5):446–452.
50. Nazari F, Safavi M, Mahmudi M. Migraine and its relation with lifestyle in women. Pain Pract. 2010;10(3):228–234.
51. Rockett FC, de Oliveira VR, Castro K, Chaves ML, Perla Ada S, Perry ID. Dietary aspects of migraine trigger factors. Nutr Rev. 2012;70(6):337–356.
52. Fonseca E, Torres-Ferrus M, Gallardo VJ, Macaya A, Pozo-Rosich P. Impact of puberty in pediatric migraine: a pilot prospective study. J Clin Neurol. 2020;16(3):416–422.
53. Kelman L. Migraine changes with age: IMPACT on migraine classification. Headache. 2006;46(7):1161–1171.
54. Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care. 2009;32(5):791–796.
55. Crowe FL, Appleby PN, Travis RC, Key TJ. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: results from the EPIC-Oxford cohort study. Am J Clin Nutr. 2013;97(3):597–603.
56. Alpay K, Ertas M, Orhan EK, Ustay DK, Lieners C, Baykan B. Diet restriction in migraine, based on IgG against foods: a clinical double-blind, randomised, cross-over trial. Cephalalgia. 2010;30(7):829–837.
57. Mitchell N, Hewitt CE, Jayakody S, et al. Randomised controlled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr J. 2011;10:85.
58. Hasselmark L, Malmgren R, Hannerz J. Effect of a carbohydrate-rich diet, low in protein-tryptophan, in classic and common migraine. Cephalalgia. 1987;7(2):87–92.
59. Bic Z, Blix GG, Hopp HP, Leslie FM. In search of the ideal treatment for migraine headache. Med Hypotheses. 1998;50(1):1–7.
60. Bic Z, Blix GG, Hopp HP, Leslie FM, Schell MJ. The influence of a low-fat diet on incidence and severity of migraine headaches. J Womens Health Gend Based Med. 1999;8(5):623–630.
61. Ferrara LA, Pacioni D, Di Fronzo V, et al. Low-lipid diet reduces frequency and severity of acute migraine attacks. Nutr Metab Cardiovasc Dis. 2015;25(4):370–375.
62. Mirzababaei A, Khorsha F, Togha M, Yekaninejad MS, Okhovat AA, Mirzaei K. Associations between adherence to dietary approaches to stop hypertension (DASH) diet and migraine headache severity and duration among women. Nutr Neurosci. 2020;23(5):335–342.
63. Nattagh-Eshtivani E, Sani MA, Dahri M, et al. The role of nutrients in the pathogenesis and treatment of migraine headaches: review. Biomed Pharmacother. 2018;102:317–325.
64. Gazerani P. Current evidence on the role of epigenetic mechanisms in migraine: the way forward to precision medicine. OBM Genetics. 2018;2:4.
65. Mathew PG, Dun EC, Luo JJ. A cyclic pain: the pathophysiology and treatment of menstrual migraine. Obstet Gynecol Surv. 2013;68(2):130–140.
66. Barnard ND, Scialli AR, Hurlock D, Bertron P. Diet and sex-hormone binding globulin, dysmenorrhea, and premenstrual symptoms. Obstet Gynecol. 2000;95(2):245–250.
67. Bunner AE, Agarwal U, Gonzales JF, Valente F, Barnard ND. Nutrition intervention for migraine: a randomized crossover trial. J Headache Pain. 2014;15:69.
68. Geppetti P, Capone JG, Trevisani M, Nicoletti P, Zagli G, Tola MR. CGRP and migraine: neurogenic inflammation revisited. J Headache Pain. 2005;6(2):61–70.
69. Ley SH, Sun Q, Willett WC, et al. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr. 2014;99(2):352–360.
70. Barbaresko J, Koch M, Schulze MB, Nothlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–527.
71. Novack V, Fuchs L, Lantsberg L, et al. Changes in headache frequency in premenopausal obese women with migraine after bariatric surgery: a case series. Cephalalgia. 2011;31(13):1336–1342.
72. Verrotti A, Agostinelli S, D’Egidio C, et al. Impact of a weight loss program on migraine in obese adolescents. Eur J Neurol. 2013;20(2):394–397.
73. Di Lorenzo C, Coppola G, Sirianni G, et al. Migraine improvement during short lasting ketogenesis: a proof-of-concept study. Eur J Neurol. 2015;22(1):170–177.
74. Bond DS, Thomas JG, Lipton RB, et al. Behavioral weight loss intervention for migraine: a randomized controlled trial. Obesity (Silver Spring). 2018;26(1):81–87.
75. Pavlovic JM, Vieira JR, Lipton RB, Bond DS. Association between obesity and migraine in women. Curr Pain Headache Rep. 2017;21(10):41.
76. Chai NC, Scher AI, Moghekar A, Bond DS, Peterlin BL. Obesity and headache: part I–a systematic review of the epidemiology of obesity and headache. Headache. 2014;54(2):219–234.
77. Di Vincenzo A, Beghetto M, Vettor R, et al. Effects of surgical and non-surgical weight loss on migraine headache: a systematic review and meta-analysis. Obes Surg. 2020;30(6):2173–2185.
78. Winter AC, Wang L, Buring JE, Sesso HD, Kurth T. Migraine, weight gain and the risk of becoming overweight and obese: a prospective cohort study. Cephalalgia. 2012;32(13):963–971.
79. Peterlin BL, Rapoport AM, Kurth T. Migraine and obesity: epidemiology, mechanisms, and implications. Headache. 2010;50(4):631–648.
80. Kristoffersen ES, Borte S, Hagen K, Zwart JA, Winsvold BS. Migraine, obesity and body fat distribution - a population-based study. J Headache Pain. 2020;21(1):97.
81. Gelaye B, Sacco S, Brown WJ, Nitchie HL, Ornello R, Peterlin BL. Body composition status and the risk of migraine: a meta-analysis. Neurology. 2017;88(19):1795–1804.
82. Ornello R, Ripa P, Pistoia F, et al. Migraine and body mass index categories: a systematic review and meta-analysis of observational studies. J Headache Pain. 2015;16:27.
83. Coppola G, Schoenen J. Cortical excitability in chronic migraine. Curr Pain Headache Rep. 2012;16(1):93–100.
84. May A, Burstein R. Hypothalamic regulation of headache and migraine. Cephalalgia. 2019;39(13):1710–1719.
85. Taib B, Bouyakdan K, Hryhorczuk C, Rodaros D, Fulton S, Alquier T. Glucose regulates hypothalamic long-chain fatty acid metabolism via AMP-activated kinase (AMPK) in neurons and astrocytes. J Biol Chem. 2013;288(52):37216–37229.
86. Hajihashemi P, Askari G, Khorvash F, Reza Maracy M, Nourian M. The effects of concurrent Coenzyme Q10, L-carnitine supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Cephalalgia. 2019;39(5):648–654.
87. Decarie-Spain L, Sharma S, Hryhorczuk C, et al. Nucleus accumbens inflammation mediates anxiodepressive behavior and compulsive sucrose seeking elicited by saturated dietary fat. Mol Metab. 2018;10:1–13.
88. Melo HM, Santos LE, Ferreira ST. Diet-derived fatty acids, brain inflammation, and mental health. Front Neurosci. 2019;13:265.
89. Borkum JM. The Migraine attack as a homeostatic, neuroprotective response to brain oxidative stress: preliminary evidence for a theory. Headache. 2018;58(1):118–135.
90. Gross EC, Klement RJ, Schoenen J, D’Agostino DP, Fischer D. Potential protective mechanisms of ketone bodies in migraine prevention. Nutrients. 2019;11:4.
91. Gross E, Putananickal N, Orsini AL, et al. Efficacy and safety of exogenous ketone bodies for preventive treatment of migraine: a study protocol for a single-centred, randomised, placebo-controlled, double-blind crossover trial. Trials. 2019;20(1):61.
92. Pakiet A, Jakubiak A, Czumaj A, Sledzinski T, Mika A. Correction to: the effect of western diet on mice brain lipid composition. Nutr Metab (Lond). 2020;17:30.
93. Pakiet A, Jakubiak A, Czumaj A, Sledzinski T, Mika A. The effect of western diet on mice brain lipid composition. Nutr Metab (Lond). 2019;16:81.
94. Soveyd N, Abdolahi M, Bitarafan S, et al. Molecular mechanisms of omega-3 fatty acids in the migraine headache. Iran J Neurol. 2017;16(4):210–217.
95. Demers G, Roy J, Machuca-Parra AI, et al. Fish oil supplementation alleviates metabolic and anxiodepressive effects of diet-induced obesity and associated changes in brain lipid composition in mice. Int J Obes (Lond). 2020;44(9):1936–1945.
96. Singh M. Mood, food, and obesity. Front Psychol. 2014;5:925.
97. Wadden TA, Tronieri JS, Butryn ML. Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol. 2020;75(2):235–251.
98. Camara-Lemarroy CR, Rodriguez-Gutierrez R, Monreal-Robles R, Marfil-Rivera A. Gastrointestinal disorders associated with migraine: a comprehensive review. World J Gastroenterol. 2016;22(36):8149–8160.
99. Arzani M, Jahromi SR, Ghorbani Z, et al. Gut-brain Axis and migraine headache: a comprehensive review. J Headache Pain. 2020;21(1):15.
100. Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation. 2019;16(1):53.
101. Wei P, Keller C, Li L. Neuropeptides in gut-brain axis and their influence on host immunity and stress. Comput Struct Biotechnol J. 2020;18:843–851.
102. Chen J, Wang Q, Wang A, Lin Z. Structural and functional characterization of the gut microbiota in elderly women with migraine. Front Cell Infect Microbiol. 2019;9:470.
103. Kechagia M, Basoulis D, Konstantopoulou S, et al. Health benefits of probiotics: a review. ISRN Nutr. 2013;2013:481651.
104. Malhotra R. Understanding migraine: potential role of neurogenic inflammation. Ann Indian Acad Neurol. 2016;19(2):175–182.
105. Dai YJ, Wang HY, Wang XJ, Kaye AD, Sun YH. Potential beneficial effects of probiotics on human migraine headache: a literature review. Pain Physician. 2017;20(2):E251–E255.
106. Martami F, Togha M, Seifishahpar M, et al. The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: a randomized double-blind controlled trial. Cephalalgia. 2019;39(7):841–853.
107. Sensenig J, Johnson M, Staverosky T. Treatment of migraine with targeted nutrition focused on improved assimilation and elimination. Altern Med Rev. 2001;6(5):488–494.
108. Naghibi MM, Day R, Stone S, Harper A. Probiotics for the prophylaxis of migraine: a systematic review of randomized placebo controlled trials. J Clin Med. 2019;8:9.
109. de Roos NM, van Hemert S, Rovers JMP, Smits MG, Witteman BJM. The effects of a multispecies probiotic on migraine and markers of intestinal permeability-results of a randomized placebo-controlled study. Eur J Clin Nutr. 2017;71(12):1455–1462.
110. van Hemert S, Breedveld AC, Rovers JM, et al. Migraine associated with gastrointestinal disorders: review of the literature and clinical implications. Front Neurol. 2014;5:241.
111. Razeghi Jahromi S, Abolhasani M, Ghorbani Z, et al. Bariatric surgery promising in migraine control: a controlled trial on weight loss and its effect on migraine headache. Obes Surg. 2018;28(1):87–96.
112. Mennigen R, Bruewer M. Effect of probiotics on intestinal barrier function. Ann N Y Acad Sci. 2009;1165:183–189.
113. Xie Y, Zhou G, Xu Y, et al. Effects of diet based on igg elimination combined with probiotics on migraine plus irritable bowel syndrome. Pain Res Manag. 2019;2019:7890461.
114. Gonzalez A, Hyde E, Sangwan N, Gilbert JA, Viirre E, Knight R. Correction for Gonzalez et al., “Migraines are correlated with higher levels of nitrate-, nitrite-, and nitric oxide-reducing oral microbes in the american gut project cohort”. mSystems. 2017;2:2.
115. Gonzalez A, Hyde E, Sangwan N, Gilbert JA, Viirre E, Knight R. Migraines are correlated with higher levels of nitrate-, nitrite-, and nitric oxide-reducing oral microbes in the American Gut Project Cohort. mSystems. 2016;1:5.
116. Thomsen LL, Olesen J. Nitric oxide in primary headaches. Curr Opin Neurol. 2001;14(3):315–321.
117. van der Kuy PH, Lohman JJ. The role of nitric oxide in vascular headache. Pharm World Sci. 2003;25(4):146–151.
118. Scuteri D, Adornetto A, Rombola L, et al. New trends in migraine pharmacology: targeting Calcitonin Gene-Related Peptide (CGRP) with monoclonal antibodies. Front Pharmacol. 2019;10:363.
119. De Matteis E, Guglielmetti M, Ornello R, Spuntarelli V, Martelletti P, Sacco S. Targeting CGRP for migraine treatment: mechanisms, antibodies, small molecules, perspectives. Expert Rev Neurother. 2020;20(6):627–641.
120. Cady RJ, Durham PL. Cocoa-enriched diets enhance expression of phosphatases and decrease expression of inflammatory molecules in trigeminal ganglion neurons. Brain Res. 2010;1323:18–32.
121. Slavin M, Bourguignon J, Jackson K, Orciga MA. Impact of food components on in vitro calcitonin gene-related peptide secretion-a potential mechanism for dietary influence on migraine. Nutrients. 2016;8:7.
122. Cady RJ, Hirst JJ, Durham PL. Dietary grape seed polyphenols repress neuron and glia activation in trigeminal ganglion and trigeminal nucleus caudalis. Mol Pain. 2010;6:91.
123. Ghorbani Z, Rafiee P, Fotouhi A, et al. The effects of vitamin D supplementation on interictal serum levels of calcitonin gene-related peptide (CGRP) in episodic migraine patients: post hoc analysis of a randomized double-blind placebo-controlled trial. J Headache Pain. 2020;21(1):22.
124. Rist PM, Buring JE, Kurth T. Dietary patterns according to headache and migraine status: a cross-sectional study. Cephalalgia. 2015;35(9):767–775.
125. Finkel AG, Yerry JA, Mann JD. Dietary considerations in migraine management: does a consistent diet improve migraine? Curr Pain Headache Rep. 2013;17(11):373.
126. Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73(2):219–234.
127. Evans EW, Lipton RB, Peterlin BL, et al. Dietary intake patterns and diet quality in a nationally representative sample of women with and without severe headache or migraine. Headache. 2015;55(4):550–561.
128. Andreeva VA, Szabo de Edelenyi F, Druesne-Pecollo N, Touvier M, Hercberg S, Galan P. Macronutrient intake in relation to migraine and non-migraine headaches. Nutrients. 2018;10:9.
129. Takeshima T, Ishizaki K, Fukuhara Y, et al. Population-based door-to-door survey of migraine in Japan: the Daisen study. Headache. 2004;44(1):8–19.
130. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. 2016;139(Pt 7):1987–1993.
131. Peroutka SJ. What turns on a migraine? A systematic review of migraine precipitating factors. Curr Pain Headache Rep. 2014;18(10):454.
132. Nowaczewska M, Wicinski M, Kazmierczak W, Kazmierczak H. To eat or not to eat: a review of the relationship between chocolate and migraines. Nutrients. 2020;12:3.
133. Marcus DA, Scharff L, Turk D, Gourley LM. A double-blind provocative study of chocolate as a trigger of headache. Cephalalgia. 1997;17(8):
134. Gibb CM, Davies PT, Glover V, Steiner TJ, Clifford Rose F, Sandler M. Chocolate is a migraine-provoking agent. Cephalalgia. 1991;11(2):93–95.
135. Moffett AM, Swash M, Scott DF. Effect of chocolate in migraine: a double-blind study. J Neurol Neurosurg Psychiatry. 1974;37(4):445–448.
136. Kokavec A. Migraine: A disorder of metabolism? Med Hypotheses. 2016;97:117–130.
137. Woldeamanuel YW, Cowan RP. The impact of regular lifestyle behavior in migraine: a prevalence case-referent study. J Neurol. 2016;263(4):669–676.
138. Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45(7):904–910.
139. Gil-Martinez A, Kindelan-Calvo P, Agudo-Carmona D, Munoz-Plata R, Lopez-de-Uralde-Villanueva I, La Touche R. [Therapeutic exercise as treatment for migraine and tension-type headaches: a systematic review of randomised clinical trials]. Rev Neurol. 2013;57(10):433–443. Spanish.
140. Molarius A, Tegelberg A, Ohrvik J. Socio-economic factors, lifestyle, and headache disorders - a population-based study in Sweden. Headache. 2008;48(10):1426–1437.
141. Liang S, Wu X, Jin F. Gut-brain psychology: rethinking psychology from the microbiota-gut-brain axis. Front Integr Neurosci. 2018;12:33.
142. Wober C, Brannath W, Schmidt K, et al. Prospective analysis of factors related to migraine attacks: the PAMINA study. Cephalalgia. 2007;27(4):304–314.
143. Peris F, Donoghue S, Torres F, Mian A, Wober C. Towards improved migraine management: determining potential trigger factors in individual patients. Cephalalgia. 2017;37(5):452–463.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
