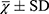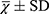Back to Journals » Cancer Management and Research » Volume 14
99Tc-Methylene Diphosphonate Treatment is Safe and Efficacious for Osteoporosis in Postmenopausal Differentiated Thyroid Cancer Patients Undergoing TSH Suppression: A Three-Center Non-Randomized Clinical Study
Authors Xie J, Yuan X, Mao W, Cai H, Gao K, Lv Z, Wang H, Ma C
Received 21 December 2021
Accepted for publication 23 February 2022
Published 5 March 2022 Volume 2022:14 Pages 995—1005
DOI https://doi.org/10.2147/CMAR.S354471
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Jianhao Xie,1,2,* XueYu Yuan,1,* Weiqing Mao,1,* Haidong Cai,1 Kejia Gao,3 Zhongwei Lv,1 Hui Wang,4 Chao Ma1
1Department of Nuclear Medicine, Tenth People’s Hospital of Tongji University, Shanghai, People’s Republic of China; 2Department of Orthopaedic, Beijing Jishuitan Hospital, Beijing, People’s Republic of China; 3Department of Nuclear Medicine, Shanghai No. 4 People’s Hospital, Shanghai, People’s Republic of China; 4Department of Nuclear Medicine, Xinhua Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhongwei Lv; Chao Ma, Tel/Fax +86-21-66302075, Email [email protected]; [email protected]
Objective: To investigate the effects of 99Tc-methylene diphosphonate (99Tc-MDP) on osteoporosis (OS) in postmenopausal patients with differentiated thyroid cancer (DTC) under thyroid stimulating hormone (TSH) suppression.
Patients and Methods: Patients (n = 142) were divided into two groups: (1) 99Tc-MDP (n = 70) and (2) alendronate (n = 72) treatments (NCT 02304757). Bone mineral density (BMD) in the lumbar spine and hip was evaluated by DXA, along with bone turnover markers, safety, and quality of life (QOL) using SF-36 at three time points: before treatment and at 6 and/or 12 months after treatment.
Results: The percentage change of BMD in total lumbar spine or hip showed no significant difference throughout the study (P > 0.025). 99Tc-MDP and alendronate treatment alone significantly increased BMD in the lumbar spine, but alendronate treatment also significantly increased BMD in total hip at 6 and 12 months, as compared with the baseline. There were no significant differences in the results of the SF-36 scores between the two treatment groups at any time during the whole study period. 99Tc-MDP significantly increased bone formation markers of osteocalcin at 6 and 12 months (P all < 0.05), PINP at 12 months (P = 0.001), and bone resorption markers of β-CTX at 6 and 12 months (p < 0.05) as compared with the alendronate treated group. No adverse event was observed in the 99Tc-MDP treatment group compared with alendronate (P = 0.014).
Conclusion: 99Tc-MDP was as efficacious as alendronate in the improvement of lumbar BMD for DTC patients with OS under TSH stimulation. 99Tc-MDP was shown to be safe and improved patients’ QOL.
Keywords: osteoporosis, 99Tc-MDP, thyroid stimulating hormone suppression, differentiated thyroid cancer
Introduction
Differentiated thyroid cancer (DTC) has become one of the most common endocrine malignancies. According to the American Thyroid Association (ATA) and Chinese Thyroid Association (CTA), most DTC patients undergo total or near total thyroidectomy, radioiodine ablation, and TSH (thyroid stimulating hormone) suppression.1,2 TSH suppression treatment is necessary for DTC as tumor cells express TSH receptors on cell membranes and respond to TSH stimulation by increasing the expression of several proteins and the rate of cell growth.3,4 However, our previous study found that excessive intake of levothyroxine (L-T4) contributed to a negative balance of bone formation and resorption resulting in bone loss.5 Postmenopausal women with DTC under TSH suppression therapy are more vulnerable to Osteoporosis (OS).6–11 OS and fractures are important comorbidities in patients with DTC, with a potential negative impact on quality of life (QOL) and survival.12 The main determinant of skeletal fragility in DTC is TSH suppression.12
99Tc-methylene diphosphonate (99Tc-MDP), a chemical compound of technetium-99 conjugated with methylene diphosphonate ([99Tc-MDP], or Yunke, Chengdu Yunke Pharmaceutical Co., Ltd., Chengdu, Sichuan, China), is an anti-inflammatory and anti-bone destruction drug patented in China, which has long been widely used and showed good efficacy for the treatment of rheumatoid arthritis (RA) (patent No. ZL94113006.1)13 and osteoporosis (patent No. ZL00100083.7) in China since 2000, respectively. Bisphosphonate drugs inhibit osteoclasts by a regulatory effect on the osteoprotegerin (OPG)/receptor activator of nuclear factor kappa‐B ligand (RANKL)/receptor activator of NF‐κB (RANK) system. 99Tc‐MDP may has the anti‐osteoclastogenic activity against RANKL‐induced osteoclast formation in vitro.14 In addition, 99Tc‐MDP promotes the differentiation and proliferation of osteoblast, and new bone formation at high concentrations.15,16 However, as a bisphosphate, little attention has been paid to its anti-OS effect for DTC under TSH suppression. The current clinical study is, as far as we know, the first one that has compared the outcome of 99mTc-MDP for OS under TSH suppression with that following alendronate treatment.
Patients and Methods
Study Design
This was an open-label, non-randomized, clinical study carried out in three centers (ClinicalTrials.gov ID: NCT02304757).
Primary End-Points
Bone mineral density (BMD) in spine lumbar and hip before, 6 and/or 12 months after treatment.
Secondary End-Points
Bone turnover markers including serum β-isomer of C-terminal telopeptide of type I collagen (β-CTX) and procollagen type 1 N-terminal propeptide (P1NP), adverse events, quality of life (QOL) by 36-item Short Form Health Status Survey questionnaire (SF-36)1 will be evaluated before, 6 and/or 12 months after treatment.
Setting and Participants
Patients were identified and enrolled during November 2015 to December 2019 from the Tenth People’s Hospital of Tongji University, Shanghai No. 4 People’s Hospital, and Xinhua Hospital, School of Medicine, Shanghai Jiaotong University. They were followed-up for a year.
Postmenopausal patients were eligible for the study if they fulfilled all the following criteria. (1) pathologically diagnosed with DTC including papillary or follicular carcinoma; (2) received a near total thyroidectomy and radioiodine treatment; (3) had BMD of the lumbar spine and/or hip was tested by dual-energy X-ray absorptiometry (DXA) at baseline, and at 6 months and 12 months; (4) undertake TSH suppression for at least one year before the study; (5) had a T-score ≤-2.5 SD for the lumbar spine, femur neck, or total hip.
We excluded patients who met the following criteria: (1) had medications for OS before TSH suppression treatment; (2) had secondary OS owing to the parathyroid or kidney disease; (3) had severe liver or kidney disease; (4) had reflux esophagitis diagnosed by gastroscope; (5) had long-term use of an immunosuppressive agent, estrogen, or estrogen receptor modulators.
This study was approved by the Institutional Review Board of Research Ethics in Shanghai Tenth People’s Hospital, Shanghai Fourth People’s Hospital, and Xinhua Hospital Ethics Committee, Affiliated to Shanghai Jiaotong University School of Medicine. All the patients were fully acquainted with their treatment and consented to participate in the clinical trial.
TSH Suppression Treatment
TSH suppression treatment was based on the risk stratification of DTC using L-T4 as recommended:1,2 (1) For patients with persistent disease, TSH suppression below 0.1 mU/L is recommended. (2) For patients free of disease but originally presented with high-risk disease, TSH suppression to from 0.1 to 0.5 mU/L is recommended. (3) For patients with low risk of recurrence, TSH suppression from 0.3 to 2 mU/L is recommended. The dose of L-thyroxine maintained stable during the study period. Free T3, free T4, and TSH were measured using a time-resolved immunofluorometric assay (Anytest, Sym-Bio Lifescience Co., Ltd, Shanghai, China).
Treatment Protocol
Patients with OS chose 99Tc-MDP or alendronate treatment after well informed the two treatment protocol.
- 99Tc-MDP treatment group: 15 mg 99Tc-MDP containing 0.15μg 99Tc was intravenously administered twice a week for 10 weeks, then once a week for 8 weeks, every 2 weeks for 22 weeks, and monthly for a further 3 months.
- Alendronate treatment group: 70 mg alendronate (Merck & Co., Darmstadt, Germany) was administered orally once a week for 12 months.
In addition, vitamin D (0.25μg) and 600mg calcium carbonate were orally administered once a day in both the treatment groups.
BMD in Spine Lumbar and Hip
DXA (v.13.20; enCORETM 2009, GE Healthcare) was used to measure BMD on L1-4 vertebral regions and the hip (femur neck, trochanter, ward, and total hip). Precision errors, established with a local normal population, were less than 1.5% for all locations at baseline, and at 6 and 12 months.
Serum Bone Turnover Markers
Serum β-CTX, P1NP, osteocalcin, and bone alkaline phosphatase (ALP) were all determined by enzyme-linked immunosorbent assay (Modular E170, Hoffmann-La Roche, Basel, Switzerland) with intra- and inter-assay coefficients of variation (CVs) of 2.7 and 3.4%.
QOL
QOL in patients with OS was measured with a SF-361 at baseline, and at 6 and 12 months. The SF-36 questionnaire includes 36 items that can be classified into the following eight health status sub-scales: physical functioning, physical role limitations, bodily pain, general health perception, vitality, social functioning, emotional role limitations, and mental health. A standardized physical component summary (PCS) and a standardized mental component score (MCS) were calculated. In SF-36, eight subscales are summary scales transformed to range 0–100, while the PCS and MCS are weighted scores. A higher score for SF-36 indicates a better QOL.
Adverse Reaction
Laboratory assays for routine blood tests, liver, and renal function were measured at baseline and 12 months. A treating physician reviewed the clinical results and any discomfort at each visit.
Study Size
The predetermined primary end point was the difference in the change in BMD of the lumbar spine between the two groups. Sixty-four patients were assumed to achieve 80% power to detect non-inferiority using a one-sided two sample t-test. The margin of non-inferiority was 1.0% percent. The true difference between the means was assumed to be 0. The significance level (alpha) of the one-sided test was 0.025. The data were drawn from a population with standard deviations of 0. Following the 10% loss of follow-up rate, the group sample size was 70 cases.
Quantitative Variables and Statistical Methods
Continuous data are expressed as the mean ± standard deviation. The independent sample t-test, Fisher exact Chi-square test in SPSS 22 was used to compare the basic information, clinical characteristics within groups at baseline, and differences in values of BMD between that at baseline, and at 6 and 12 months after treatment. Differences in QOL according to the SF-36 questionnaire, bone turnover markers, and other laboratory results were determined using a paired t-test (one-sided tests), Wilcoxon paired t-test, and Mann–Whitney U-test. Fisher exact Chi-square test was used to compare adverse events.
Results
Clinical Characteristics of Participants
In total, 152 postmenopausal DTC patients with OS under TSH suppression were enrolled. Five patients were excluded and five patients were lost during follow-up. Out of the 142 included patients, 70 were treated with 99Tc-MDP and 72 were treated with alendronate (Figure 1). The age, weight, BMI, TSH values, duration of TSH suppression, BMD, etc. at baseline are listed in Table 1 and showed no significant difference (P > 0.05).
 |
Table 1 Patients’ Clinical Characteristics |
 |
Figure 1 Study flow chart. |
Percent Changes of BMD and Serum Bone Turnover Markers
Between group comparisons of the percentage change in BMD of the total lumbar spine were not significant. However, 99Tc-MDP and alendronate treatment alone significantly increased total lumbar spine BMD at 6 months and 12 months, respectively, as compared with baseline. Alendronate treatment also significantly increased BMD in total hip at 6 and 12 months as compared with baseline (Table 2 and Figure 2).
 |
Table 2 Areal Bone Mineral Density (BMD, g/cm2) and Mean Percent Change (%, |
The mean value and mean percent change in serum β-CTX, PINP, osteocalcin, and ALP levels during the 12-month study period are shown in Figure 3 and Table 3. 99Tc-MDP significantly increased bone formation markers of osteocalcin (P < 0.001) and PINP at 12 months (P = 0.0005) as compared with the alendronate treated group. A significant decrease was found in β-CTX at 6 months (P = 0.0005) and 12 months (P < 0.001), and in ALP at 6 months (P = 0.0045) in the alendronate treated group as compared with the 99Tc-MDP treatment group.
 |
Table 3 Changes in Bone Metabolism Markers (ng/mL) and Mean Percent Change (%, |
QOL
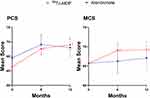
There were no significant differences in the results of the SF-36 scores between the two treatment groups at any time during the whole study period (Figure 4). The 99Tc-MDP treated group exhibited increased PCS and MCS at 6 months (P = 0.008 and 0.0055, respectively) and 12 months (P = 0.001 and 0.013, respectively), as compared with that before treatment. However, no significant difference was found in PCS and MCS scores in the alendronate group during the study (Figure 4; P > 0.05).
Safety
No significant difference was found in terms of liver and renal function in the two groups (P > 0.025) before or after treatment (Table 4). A significant increase was found in the incidence of upper gastrointestinal (GI) adverse events in the OS patients treated with alendronate (6/72) compared with 99Tc-MDP (0/70) (Fisher test, P = 0.014). In the 6/72 patients with GI adverse events treated with alendronate, two cases had abdominal pain and four had dyspepsia, which did not need further medication. No bone fractures were found in the two groups during the one-year follow-up.
 |
Table 4 Changes in Routine Clinical Chemistry Parameters |
Discussion
In the 1990s, some of nuclear medicine physicians found that 99mTc-MDP, a bone imaging agent, had an analgesic effect for rheumatoid patients. Based on the information described above, Professor Maoliang Li suggested that it should be technetium [99Tc],2 a trace element with a half-life of 2.13X105 years emitting low energy beta particles contributed to the therapeutic effect. The specific radioactivity of 99Tc is 625.3Bq/μg, and the final decay product is stable isotope 99Ru. Later study found that 99Tc had anti-inflammatory and antirheumatic effects by decreasing serum levels of tumor necrosis factor (TNF) and interleukin-1, prostaglandins E1-2 and histamine, thereby promoting pain relief of knee.3 Moreover, it’s also possible that phosphorus (phosphine) plays a role in the therapeutic effect of the imaging agent. However, there may be the possibility of radioactive effect of 99Tc on OS which has not been investigated yet. Chinese studies17,18 reported that 99Tc-MDP improved the back pain and increased the lumbar BMD. The effect of 99Tc-MDP on OS in postmenopausal DTC patients under TSH suppression is not clear and investigated in the study.
Results indicated that 99Tc-MDP was as efficacious as alendronate in the improvement of lumbar BMD for DTC patients with OS under TSH stimulation. 99Tc-MDP was safe and improved patients’ QOL. 99Tc-MDP is a novel bisphosphate derivative and has been used in the treatment of rheumatoid arthritis in China since 2000.19 Nitrogen-containing bisphosphonates (N-BPs), such as alendronate, are the standard treatment for OS,12,20,21 and alendronate was used for comparison in the current study. To our knowledge, the current study is the first to compare the outcome of these two treatment modalities in postmenopausal women with DTC and OS under TSH suppression. As expected, no difference in the percent change of BMD was found between the two treatment groups. However, both 99Tc-MDP and alendronate alone significantly increased both BMD in the lumbar spine, while alendronate also increase the BMD in the total hip. Alendronate increases BMD by inhibiting the effects of bone resorption22,23 characterized by the significant decrease in β-CTX in our study. Increased resorption markers in the 99Tc-MDP group may indicate the worse anti-osteoclastogenic activity as compared with alendronate which should be clarified in large sample size and long term. The reported mechanisms of 99Tc-MDP may be owing to the elevation of osteogenic capacity of mesenchymal stem cells, decreased adipogenic differentiation capacity,24 inducement of osteoblast proliferation and differentiation, inhibition of differentiation.14,15,25
The duration of exposure to suppressed TSH values was found to be an important determinant of skeletal health. In the current study, TSH suppression was at least 1 year before the study and showed no significant difference among these groups. TSH suppression therapy is also associated with an increased risk of fragility fractures and fracture-related deaths mainly in postmenopausal women and the elderly.12 Fortunately, no fragility fractures were observed in our patients. And radiological vertebral fractures were found to be a frequent complication of long-term TSH-suppressive therapy even in patients with BMD T-score values above −2.5 SD.
Potential safety issues should be considered when bisphosphonates are used because N-BPs cause rare yet serious side effects, such as atypical femoral fractures, osteonecrosis of the jaw,12,20,26–28 and atrial fibrillation, which may be relatively increased in patients with subclinical hyperthyroidism.29 In the current study, alendronate produced a significant increase in the incidence of upper gastrointestinal adverse events. However, no adverse reactions were found following 99Tc-MDP treatment. Therefore, 99Tc-MDP treatment was deemed to be safe for patients with OS. The study also showed that QOL was similar in both treatment groups. However, 99Tc-MDP significantly improved both the physical and mental QOL as compared with that before treatment. The improvement in QOL was somewhat slower in the alendronate treated group but the difference was not significant.
Oral administration of alendronate is much easier than intravenous administration of 99Tc-MDP. However, alendronate needs to be administered on empty stomach in a specific way. The intravenous administration of 99Tc-MDP may be advantageous over alendronate to eliminate the risk of improper administration, especially in cases of poor compliance, which can be the case in some elderly patients.
There were some limitations in our study such as the non-randomized nature. However, the clinical characteristics of patients in both groups were very similar, which minimizes the significance of the lack of randomization. The study was also not blinded and the choice of treatment was based on the conscious decision of the participants, which could result in biased answers in the SF-36 questionnaire. The effects of 99Tc-MDP on OS should and will be observed during a long-term follow-up in large population.
In conclusion, 99Tc-MDP proved to be as efficacious as alendronate in the improvement of lumbar BMD for DTC patients with OS under TSH stimulation. 99Tc-MDP appeared to be safe and improved patients’ QOL.
Data Sharing Statement
No further data will be shared. This study was approved by the Institutional Review Board of Research Ethics in Shanghai Tenth People’s Hospital, Shanghai Fourth People’s Hospital, and Xinhua Hospital Ethics Committee, Affiliated to Shanghai Jiaotong University School of Medicine. Our study complies with the Helsinki Declaration of 1975, as revised in 2000. The authors will obtain the copyright permission to use the figure if the revised manuscript is accepted by the Editor for publication. The data will be accessible through the clinical trial website and in 5 years.
Acknowledgments
We acknowledge Professor Rui Wang and Qin Yingyi from Department of Statistics, The Second Military Medical University for their statistical guidance.
Funding
This work was supported by the National Natural Science Fund (grant numbers 82171974, 81771859, and 82071964), the Shanghai Health Bureau Fund (grant 202040085), the Clinical Research Plan of SHDC (grant number 16CR3114B), and Shanghai Shenkang Three-year Action Project (grant number: SHDC2020CR2054B).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020
2. Gao M. Diagnosis and treatment guideline for thyroid nodules and differentiated cancer. Cancer Clin China. 2012;17:1249–1272.
3. Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid. 2010;20(2):135–146. doi:10.1089/thy.2009.0311
4. Brabant G. Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab. 2008;93(4):1167–1169. doi:10.1210/jc.2007-2228
5. Huo Y, Wang D, Wu S, Wang H, Ma C. The impact of TSH suppression treatment on bone mineral density in postmenopausal patients with differentiated thyroid cancer. Chin J Nucl Med Mol Imaging. 2017;37(4):212–215.
6. Diamond T, Nery L, Hales I. A therapeutic dilemma: suppressive doses of thyroxine significantly reduce bone mineral measurements in both premenopausal and postmenopausal women with thyroid carcinoma. J Clin Endocrinol Metab. 1991;72(6):1184–1188. doi:10.1210/jcem-72-6-1184
7. Heemstra KA, Hamdy NA, Romijn JA, Smit JW. The effects of thyrotropin-suppressive therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Thyroid. 2006;16(6):583–591. doi:10.1089/thy.2006.16.583
8. Karner I, Hrgović Z, Sijanović S, et al. Bone mineral density changes and bone turnover in thyroid carcinoma patients treated with supraphysiologic doses of thyroxine. Eur J Med Res. 2005;10(11):480–488.
9. Kim MK, Yun KJ, Kim MH, et al. The effects of thyrotropin-suppressing therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Bone. 2015;71:101–105. doi:10.1016/j.bone.2014.10.009
10. Kung AW, Lorentz T, Tam SC. Thyroxine suppressive therapy decreases bone mineral density in post-menopausal women. Clin Endocrinol. 1993;39(5):535–540. doi:10.1111/j.1365-2265.1993.tb02405.x
11. Sugitani I, Fujimoto Y. Effect of postoperative thyrotropin suppressive therapy on bone mineral density in patients with papillary thyroid carcinoma: a prospective controlled study. Surgery. 2011;150(6):1250–1257. doi:10.1016/j.surg.2011.09.013
12. Cellini M, Rotondi M, Tanda ML, et al. Skeletal health in patients with differentiated thyroid carcinoma. J Endocrinol Invest. 2021;44(3):431–442. doi:10.1007/s40618-020-01359-6
13. Fu Q, Feng P, Sun LY, et al. A double-blind, double-dummy, randomized controlled, multicenter trial of 99Tc-methylene diphosphonate in patients with moderate to severe rheumatoid arthritis. Chin Med J. 2021;134(12):1457–1464. doi:10.1097/CM9.0000000000001527
14. Gong W, Dou H, Liu X, Sun L, Hou Y. Technetium-99 conjugated with methylene diphosphonate inhibits receptor activator of nuclear factor-κB ligand-induced osteoclastogenesis. Clin Exp Pharmacol Physiol. 2012;39(10):886–893. doi:10.1111/j.1440-1681.2012.12006.x
15. Chen J, Lan Y, He Y, et al. 99Tc-MDP-induced human osteoblast proliferation, differentiation and expression of osteoprotegerin. Mol Med Rep. 2017;16(2):1801–1809. doi:10.3892/mmr.2017.6839
16. Shen S, Wang W, Yang C, Xu B, Zeng L, Qian Y. Effect of technetium-99 conjugated with methylene diphosphonate ((99) Tc-MDP) on OPG/RANKL/RANK system in vitro. J Oral Pathol Med. 2019;48(2):129–135.
17. Duan Y. Clinical analysis of yunke in the treatment of primary osteoporosis. Chin Med Guide. 2011;9(18):259–260.
18. Guo Z, Zhang M, Zhang C. Clinical observation of yunke in treating 93 cases of postmenopausal osteoporosis. Chin J Osteoporos. 2012;18(4):358–360.
19. Liu H, Guo H, Guo S, Wang J, Ye Y, Ma C. Novel treatment of 99Tc-MDP improves clinical and radiographic results for patients with osteochondral lesions of the talus. Q J Nucl Med Mol Imaging. 2019;63(2):199–206. doi:10.23736/S1824-4785.16.02872-7
20. Favus MJ. Bisphosphonates for osteoporosis. N Engl J Med. 2010;363(21):2027–2035. doi:10.1056/NEJMct1004903
21. Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2005;353(6):595–603. doi:10.1056/NEJMcp043801
22. D’Amelio P, Grimaldi A, Cristofaro MA, et al. Alendronate reduces osteoclast precursors in osteoporosis. Osteoporos Int. 2010;21(10):1741–1750. doi:10.1007/s00198-009-1129-1
23. Siebelt M, Waarsing JH, Groen HC, et al. Inhibited osteoclastic bone resorption through alendronate treatment in rats reduces severe osteoarthritis progression. Bone. 2014;66:163–170. doi:10.1016/j.bone.2014.06.009
24. Zhao Y, Wang L, Liu Y, et al. Technetium-99 conjugated with methylene diphosphonate ameliorates ovariectomy-induced osteoporotic phenotype without causing osteonecrosis in the jaw. Calcif Tissue Int. 2012;91(6):400–408. doi:10.1007/s00223-012-9649-7
25. Shi L, Ning Y, Xu L, Li J, Zhang X. Technetium-99 conjugated with methylene diphosphonate ameliorates glucocorticoid induced osteoporosis by inhibiting osteoclastogenesis. Biomed Res Int. 2018;2018:7902760. doi:10.1155/2018/7902760
26. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555–1565. doi:10.1210/jc.2009-1947
27. Bi Y, Gao Y, Ehirchiou D, et al. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am J Pathol. 2010;177(1):280–290. doi:10.2353/ajpath.2010.090592
28. Khosla S, Cauley JA, Compston J, et al. Addressing the crisis in the treatment of osteoporosis: a path forward. J Bone Miner Res. 2017;32(3):424–430. doi:10.1002/jbmr.3074
29. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. doi:10.1056/NEJMoa067312
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.