Back to Journals » Cancer Management and Research » Volume 12
99mTc-Methylene Diphosphonate Uptake in Soft Tissue Tumors on Bone Scintigraphy Differs Between Pediatric and Adult Patients and Is Correlated with Tumor Differentiation
Authors Liu S , Xie J, Yu F, Cai H, Wu F, Zheng H, Ma C, Lv Z, Wang H
Received 9 December 2019
Accepted for publication 21 March 2020
Published 3 April 2020 Volume 2020:12 Pages 2449—2457
DOI https://doi.org/10.2147/CMAR.S241636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Simin Liu,1,* Jianhao Xie,2,* Fei Yu,1 Haidong Cai,1 Fengyu Wu,3 Hui Zheng,1 Chao Ma,1 Zhongwei Lv,1 Hui Wang4
1Department of Nuclear Medicine, Tenth People’s Hospital of Tongji University, Shanghai, People’s Republic of China; 2Medical School of Soochow University, Suzhou, People’s Republic of China; 3Department of Nuclear Medicine, Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 4Department of Nuclear Medicine, Xinhua Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao Ma; Zhongwei Lv
Department of Nuclear Medicine, Tenth People’s Hospital of Tongji University, Yanchangzhong Road 301, Shanghai 200072, People’s Republic of China
Tel +8621-66302075
Email [email protected]; [email protected]
Objective: To analyze the difference in 99mTc-methylene diphosphonate (MDP) uptake on bone scintigraphy in extraosseous soft tissue tumors between children and adults and the correlation between tracer uptake and tumor differentiation and histopathology.
Methods: Patients with neoplasms with MDP uptake were retrospectively identified. Based on histopathology, tumors were categorized as epithelial malignant tumors, mesenchymal tumors, blastomas and germ cell tumors. The degree of radioactivity accumulation in lesions relative to the uptake in ribs and sternum or spine was classified as “+”, “++” and “+++”. The results were compared between children and adults. The correlations between MDP uptake in soft tumors and tumor differentiation and pathology were investigated.
Results: Extraosseous soft tissue tumors that accumulated MDP were found in 33 children and 31 adults. In children, neuroblastoma was the most common extraosseous soft tissue tumor that accumulated MDP; in adults, MDP uptake was mostly found in lung cancer. MDP uptake in pediatric soft tissue tumors was higher than that in adults. MDP uptake in extraosseous soft tissue tumors with different histopathologic classifications was significantly different among 64 patients. In 41 patients with available tumor differentiation data from histopathology, MDP uptake in low or poorly differentiated soft tumors was higher than that in the moderately or well-differentiated lesions. Necrosis and/or calcifications were showed in most of pediatric and adult neoplasms.
Conclusion: Significant elevations in MDP uptake in extraosseous soft tissue tumors are associated with poorly differentiated tumors in both children and adults. The mechanism of bone tracer uptake in pediatric and adult neoplasms was mostly related to necrosis and/or necrosis and calcification. The extraosseous soft tissue tumors with MDP uptake in pediatric patients were different from those in adults. In addition, consistent with the inherent degree of tumor malignancy, MDP uptake in children was higher than that in adults.
Keywords: pediatric, adult, extraosseous soft tissue tumor, differentiation, bone scintigraphy, MDP
Introduction
Bone scintigraphy with 99mTc-MDP is the most commonly performed nuclear medicine procedure to assess primary bone tumors, skeletal metastases, infections and sites of skeletal trauma. MDP localizes to bone by ion exchange and chemisorption to the surface of hydroxyapatite crystals. Sometimes, certain abnormal processes involving extraosseous organs or soft tissue tumors can also cause the accumulation of MDP on bone scintigraphy. There have been many case reports about this abnormality.1,2 Cases of pediatric extraosseous soft tissue tumors that accumulated MDP, such as neuroblastoma, osteosarcoma and rhabdomyosarcoma, were reported,3,4 but a large-scale summary, especially a recent summary, has not been reported for blastomas other than neuroblastoma. Many pediatric tumors were found to have MDP uptake on bone scans in our clinical practice. We hypothesized that there may be differences in MDP uptake in extraosseous soft tissue tumors between children and adults, which may also be correlated with tumor characteristics and differentiation. Currently, no studies have addressed these questions. Therefore, we retrospectively collected data from pediatric and adult patients with MDP uptake in neoplasms from three nuclear medicine centers to investigate the correlation of MDP uptake characteristics in extraosseous soft tissue tumors with tumor differentiation.
Patients and Methods
Research Object
Inclusion criteria: ① patients with extraosseous soft tissue tumors that accumulated MDP on their pre-treatment scans; ② extraosseous soft tissue tumors were confirmed by SPECT/CT, other correlative imaging examinations and laboratory tests, such as CT, MRI, ultrasound, and blood calcium; ③ all tumors had histopathological diagnoses; and ④ pediatric tumors often occur in children younger than 15 years of age; therefore, according to Doll’s revised International Standard Population, individuals aged 14 years and younger were defined as children.5 In our study, patients 14 years and younger were placed in the pediatric group, and patients older than 14 years were placed in the adult group.
Exclusion criteria: ① normal distribution of radioactivity in the urinary system or abnormal distribution due to urinary tract obstruction, such as stones; ② symmetrical distribution of radioactivity in the breast or diffuse distribution of radioactivity in the contralateral breast after breast surgery; ③ thyroid radioactivity caused by technical problems such as drug labeling; ④ background signal caused by technical problems; ⑤ lymph node radioactivity due to the leakage of the injection agent; and ⑥MDP uptake in extraosseous benign soft tissue tumors caused by fracture, infection, etc.
Radiotracer, Quality Control and Instruments
99mTc-MDP injections were supplied by Shanghai Atomics Kexing Pharmaceutical Co. Ltd. (radiochemical purity >95%). All acquisitions were performed with a GE Infinia Hawkeye (WI USA), a Philips Precedence 16 SPECT/CT (Philips, Netherlands) equipped with low energy, high resolution collimators (10% window width, peak energy 140 keV, and 256×1024 matrix). The imaging processing systems were from GE Medical Systems, Milwaukee, WI.
Scanning Methods
The patients underwent whole-body bone scans 2~3 h after the intravenous administration of MDP (adult 370~740 MBq, children 7.4 MBq/kg). Children who could not cooperate were given 10% chloral hydrate orally (0.5 mL/kg). Standard whole-body anterior and posterior sweep images were acquired with a scan speed of 10~20 cm/min. If necessary, lateral projection or SPECT/CT tomography fusion imaging was performed to clearly show the anatomical location of extraosseous soft tissue. When urine contamination was suspected, a local scan was performed after cleaning or removing the contaminated clothing.
Image Evaluation
Visual analysis was performed by two nuclear medicine physicians based on the highest degree of radioactivity accumulation in extraosseous soft tissue tumors. The standard rules were as follows. The radioactivity distribution of the lesion area was defined as “+” when it was higher than the normal lower limb soft tissue and lower than the normal rib; when the radioactivity of the lesion was equal to or higher than the normal rib but lower than the normal sternum or spine, it was defined was “++”; when the lesion radioactivity was equal to or higher than the normal sternum or spine, it was defined as “+++”.
Tumor Classification and Statistical Analysis
All pathological results were classified by the 9th edition of Pathology,6 which was published by People’s Medical Publishing House, and the 4th edition of the WHO Classification of Tumors.7 Because some pathological types were rare, we grouped all cases according to the general classification of tumors. IBM SPSS 24.0 software was used for statistical analysis. Enumeration data are presented as a comparison of two or more sets of ranked data and Chi-Pearson tests. P˂0.05 was considered statistically significant.
Results
Basic Characteristics of Pediatric and Adult Patients with MDP Uptake in Extraosseous Soft Tissue Tumors
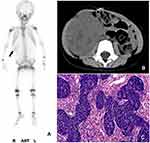
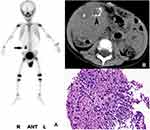
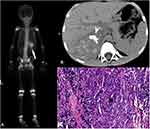
Among the patients who underwent bone scintigraphy from 2008 to 2018 at three centers, which included 1,025 children and 9,167 adults, 64 consecutive patients were enrolled in this study, including 33 patients in the pediatric group and 31 patients in the adult group. The serum calcium of all patients, which was measured within one week before or after the bone scan, was in the normal range. Among the 33 pediatric patients, the ages ranged from 3 months to 10 years, with a median age of 3 years, and there were 21 males and 12 females. The preliminary imaging results of the pediatric group are shown in Table 1 (Figures 1–3). Combined with other related examinations, including CT, MRI, ultrasound and pathological reports, which were obtained within 2 months before or after the bone scan, except for a right cervical malignant extrarenal rhabdoid tumor and vascular tumor in left thigh, the summary of the main imaging features is shown in Table 2. Twenty-eight patients (28/33, 84.8%) showed varying degrees of necrosis and/or calcification in the lesions. Three patients had pulmonary metastases from osteosarcoma containing the osteoid matrix.
 |
Table 1 Characteristics of MDP Uptake in Pediatric Soft Tissue Tumors (N=33) |
 |
Table 2 Main Imaging Features of Pediatric and Adult Soft Tissue Tumors |
Among the 31 adult patients, the ages ranged 40 to 80 years, with a median age of 59.3 years, and there were 19 males and 12 females. The general imaging results are summarized in Table 3. Among 31 patients, there were 11 cases of primary lung cancer, including 6 cases of poorly differentiated adenocarcinoma, 4 cases of moderately differentiated squamous cell carcinoma, and 1 case of small-cell lung cancer. There were 9 cases of liver metastases, which were derived from moderately differentiated adenocarcinoma in the sigmoid colon and rectum (2 cases each), moderately differentiated stomach adenocarcinoma, moderately differentiated squamous cell carcinoma in the gastric cardia, moderately differentiated esophageal squamous cell carcinoma, invasive lobular breast carcinoma and invasive ductal breast carcinoma (one case each). There were 3 cases of cervical lymph node metastasis from moderately differentiated esophageal squamous cell carcinoma, moderately differentiated ovarian serous papillary cystadenocarcinoma and poorly differentiated adenocarcinoma of the parotid gland. There were 2 cases of adrenal metastases from poorly differentiated adenocarcinoma of the lung and invasive lobular carcinoma of the breast. In addition, there was moderately differentiated intrahepatic cholangiocarcinoma (ICC), brain metastasis from poorly differentiated lung adenocarcinoma, chest wall metastasis from moderately differentiated renal clear cell carcinoma, abdominal metastasis from moderately differentiated ovarian serous papillary cystadenocarcinoma, thyroid follicular adenocarcinoma, and left thigh malignant fibrous histiocytoma (one case each). The main imaging features are also shown in Table 2. Necrosis and/or calcification was found in 22 cases (22/33, 71.0%).
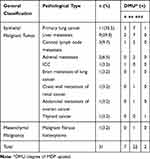 |
Table 3 Characteristics of MDP Uptake in Adult Soft Tissue Tumors (N=31) |
Comparison of MDP Uptake in Extraosseous Soft Tissue Tumors
Pediatric Extraosseous Soft Tissue Tumors
Based on the 4th edition of the WHO Classification of Tumors, 33 children were generally classified as having blastoma that originated from naive cells (such as neuroblastoma, hepatoblastoma, Wilms’ tumor and pancreatoblastoma), mesenchymal tissue tumors (such as pulmonary metastases from osteosarcoma), a malignant extrarenal rhabdoid tumor and a vascular tumor, and germ cell tumors. The degree of MDP uptake in the different pathological types of soft tissue tumors in the pediatric group is shown in Table 4.
 |
Table 4 The Degree of MDP Uptake in Pediatric Soft Tissue Tumors |
Adult Extraosseous Soft Tissue Tumors
According to the 9th edition of Pathology published by People’s Medical Publishing House, malignant tumors are usually categorized into epithelial malignant tumors (such as squamous cell carcinoma, adenocarcinoma, basal cell carcinoma and urothelial carcinoma) and mesenchymal malignant tumors (MMTs). The tumors in the adult group were generally classified into three categories on the basis of these classification principles. The degree of MDP uptake in the different pathological types of soft tissue tumors in the adult group is provided in Table 5.
 |
Table 5 The Degree of MDP Uptake in Adult Soft Tissue Tumors |
Differences in MDP Uptake in Extraosseous Soft Tissue Tumors Between Children and Adults
Table 6 showed the differences in MDP uptake in extraosseous soft tissue tumors between children and adults was statistically significant, the degree of MDP in pediatric group was higher than that in adult group (Z=2.760, P=0.006).
 |
Table 6 Comparison of MDP Uptake in Pediatric and Adult Soft Tissue Tumors |
The Correlation of MDP Uptake with Pathological Types and Tumor Differentiation
Correlation of MDP Uptake with the Pathological Types
There was a significant difference in the degree of MDP uptake in 64 patients with different pathological types of soft tissue tumors (H=27.368, P=0.000). The details were analyzed in Table 7.
 |
Table 7 The Degree of MDP Uptake in 64 Cases with Different Soft Tissue Tumors |
Correlation of MDP Uptake with Tumor Differentiation
Twenty-six patients in the adult group had specific pathological differentiation data available, which included 16 cases of moderately differentiated tumors (16/26, 61.5%; 6 cases of “+” and 10 cases of “++”) and 10 cases of poorly differentiated tumors (10/26, 38.5%; 9 cases of “+” and one case of “+++”). In the pediatric group, there were 15 cases of neuroblastoma with specific pathological differentiation data available, which included 6 cases of differentiated neuroblastoma (6/15, 40%; one case of “+”, 4 cases of “++” and one case of “+++”) and 9 cases of poorly differentiated neuroblastoma (9/15, 60%; one case of “+”, one case of “+” and 7 cases of “+++”). A total of 41 patients were divided into two groups: 22 cases of moderately differentiated or differentiated tumors (22/41, 53.7%) and 19 cases of poorly differentiated tumors (19/41, 46.3%). Then, the correlation of MDP uptake with tumor differentiation was analyzed in Table 8.
 |
Table 8 Difference in MDP Uptake in Soft Tissue Tumors with Different Differentiation Levels |
Discussion
The uptake of MDP in extraosseous soft tissue can usually be seen as a result of technical factors or artifacts, the physiological uptake in normal organs and neoplastic uptake.8 Cases of pediatric extraosseous soft tissue tumors that accumulated MDP were previously reported,3,4 but no further systematic analysis was made. The difference in the neoplastic uptake of MDP between pediatric and adult patients had not been investigated. Our research found that neuroblastoma was the most common extraosseous soft tissue tumor with MDP uptake (15/33, 45.5%), followed by Wilms’ tumor (6/33, 18.2%) and hepatoblastoma (4/33, 12.1%). Otherwise, MDP uptake was mostly found in lung cancer (11/31, 35.5%) in adults, followed by liver metastases from the gastrointestinal tract and breast (9/31, 29.0%), which was basically consistent with previous reports.9 Our study also confirmed that the extraosseous soft tissue tumors that accumulated MDP were likely to be malignant. Altogether, neoplastic uptake was observed in 63 pediatric (32/33) and adult (31/31) malignant tumors, with the exception of one pediatric benign vascular tumor. However, because most patients that underwent bone scintigraphy had a history of cancer or suspected tumors, the population enrolled in this study was biased to a certain extent, which might have resulted in a higher proportion of malignant tumors.
Previous studies suggested that the mechanisms of the neoplastic uptake of MDP were mainly related to necrosis and/or calcification, a rich blood supply and the osteoid matrix in tumors,9 which was also supported by our study based on the findings of CT, MRI, ultrasound etc.: 28 (28/33, 84.8%) pediatric and 22 (22/31, 71.0%) adult tumors had varying degrees of necrosis and/or calcification in the lesions. Namely, when cells undergo necrosis, the mitochondria and endoplasmic reticulum in the cytoplasm swell to form vacuoles, which causes the accumulation of amorphous calcium densities in the mitochondrial matrix. If necrotic cells and cell debris are not cleared in time, they will absorb calcium salts and other minerals, resulting in dystrophic calcification. The other possible mechanism of MDP accumulation is that there is increased blood flow to the neoplasm or abnormal calcium metabolism, which was affected by paraneoplastic syndrome;10 this could explain the 11 (11/64,17%) neoplasms that exhibited the uptake of a bone tracer in our study.
In addition, our results suggested that the degree of MDP uptake in pediatric extraosseous soft tissue tumors was higher than that in adult tumors, which was correlated with the higher incidence and the density of calcifications and the extent of necrosis in pediatric tumors. Most pediatric malignant soft tissue tumors originate from highly malignant immature cells that grow fast, have a large volume, and tend to form a large amount of ischemic necrosis and dystrophic calcification. Therefore, the increased MDP uptake in pediatric malignant soft tumors corresponded well to the high degree of malignancy, the higher density of calcification and the large necrotic area.
The characteristics of calcifications in pediatric tumors are different from those in adult tumors. Calcifications in pediatric tumors mostly manifest as plaque, eggshell-like, flaky or cloudy calcifications. MDP uptake was found in 9 adult hepatic metastases because of tumor calcification and/or necrosis and in 7 adult metastatic adenocarcinomas from the gastrointestinal tract and breast. These kinds of adenocarcinomas, which have a mucinous component, possess a glycoprotein that is biochemically similar to ossifying cartilage that binds calcium salts, which accumulate MDP.9,11-13 Lymph node metastases or hepatic metastases of these types of tumors easily bind calcium salts, which manifest as tiny, grit-like calcifications on CT, showing a lower degree of MDP uptake. The highest MDP uptake was found in an adult with thyroid follicular adenocarcinoma, which showed numerous irregular calcifications and high calcium density on CT images.
Our results from 41 patients with an available, specific tumor differentiation diagnosis showed that the MDP uptake of low- or poorly differentiated soft tissue tumors was higher than that of moderately or well-differentiated tumors, which suggested that the degree of neoplastic MDP uptake might be associated with tumor differentiation. This observation could also be explained by the characteristics of the tumors. Poorly differentiated tumors tend to have large areas of necrosis and subsequently develop high-density calcifications, as shown by our study. In contrast, the differentiated neuroblastomas had less necrosis and spotty, cloudy, and small amounts of calcifications or low-density calcification on the CT images. Similarly, breast cancer and thyroid cancer with tiny, grit-like calcifications were associated with poor differentiation and high malignancy, as previously reported.14,15
Until now, there have been few reports about the relationship between MDP uptake in extraosseous soft tissue tumors and their histopathological types. Overall, our results suggested that the degree of MDP uptake in epithelial malignant tumors and blastomas was higher than that in mesenchymal tumors and germ cell tumors, which may suggest that there is a correlation between the histopathological types and MDP uptake. However, no significant correlation was found in the degree of neoplastic MDP uptake and the histopathological types in children and adults. This lack of correlation may have been due to the similar characteristics of the malignant tumors, which grow fast and were large, and the limited number of included patients. A large sample size is needed to further verify these results. Most adult tumors that accumulated MDP were adenocarcinoma (23/31, 74.2%) and squamous cell carcinoma (7/31, 22.6%), which were derived from the cancers of the parotid gland, thyroid gland, lung, breast, digestive tract, liver, kidney and ovary. The involved organs or tissues also included the brain, chest wall, lymph node, adrenal gland and peritoneum, which was consistent with previous reports.9 However, in regard to MDP uptake, more adenocarcinoma than squamous cell carcinoma displayed MDP uptake in our patient sample (74.2% VS 22.6%), and the degree of MDP uptake in adenocarcinoma, which is characterized by a rich blood supply and a mucinous component that is able to accumulate calcium salts, was higher than that in squamous cell carcinoma in adults that had no obvious calcifications but had necrosis.
Conclusion
The significantly increased MDP uptake of extraosseous soft tissue tumors is associated with poor differentiation in both children and adult tumors. Extraosseous soft tissue tumors with MDP uptake differ among pediatric and adult patients. In addition, consistent with the inherent tumor malignancy, MDP uptake is higher in children than in adults.
Abbreviations
MDP, methylene diphosphonate; EMTs, epithelial malignant tumors; CT, computed tomography; MRI, magnetic resonance imaging; MMTs, mesenchymal malignant tumors; ICC, intrahepatic cholangiocarcinoma; DMU, degree of MDP uptake.
Consent for Publication
Authors have obtained written informed consent to publish the images from the participants or parent/guardian.
Ethics and Consent Statements
Written informed consent was obtained from all participants, a parent or legal guardian provided this consent for any patient under the age of 18 years in this study, and the study had gotten the permission of the ethics committee of Tenth People’s Hospital of Tongji University.
Acknowledgments
The authors wish to thank the anonymous referees and editors of this special issue for their constructive comments. The authors thank Prof. Shuyao Zuo (Qingdao University), Dr. Chao Li (Shanghai Jiaotong University, Xinhua Hospital) for their support.
Author Contributions
Simin Liu and Jianhao Xie contributed equally to the work, Simin Liu contributed to the conception, design, the interpretation of the data and drafting the manuscript. Jianhao Xie contributed to the acquisition, analysis of data and drafting the manuscript. Fengyu Wu contributed to design the manuscript, acquire and analyze the data. Hui Wang contributed to the conception, the acquisition of the data. Haidong Cai and Hui Zheng contributed to the interpretation and analysis of the data. Fei Yu contributed to some revision works. Chao Ma and Zhongwei Lv contributed to revising of the manuscript critically for important intellectual content. In addition, Chao Ma contributed to the final manuscript approval for submission and publication. All in all, all authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was funded by the National Natural Science Fund (grant number 81771859), Shanghai Science and Technology Commission Project (grant number 18401901300), and Jiangsu Student’s Platform for innovation and entrepreneurship training program (grant number 2019suda052), and the Clinical Research Plan of SHDC (grant number 16CR3114B) funded the design of the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Verma S, Kumar N, Kheruka SC, Gambhir S. Extraosseous (99m)Tc-methylene diphosphonate uptake on bone scan: unusual scenario. Indian J Nucl Med. 2016;31(4):280–282. doi:10.4103/0972-3919.190799.
2. Castaigne C, Martin P, Blocklet D. Lung, gastric, and soft tissue uptake of Tc-99m MDP and Ga-67 citrate associated with hypercalcemia. Clin Nucl Med. 2003;28(6):467–471. doi:10.1097/01.RLU.0000067505.24908.5C
3. Franco A, Henderson PR, McDonough CH. Unusual concentration of Tc-99m methylendiphosphonate in rhabdomyosarcoma. J Radiol Case Rep. 2012;6(9):29–34. doi:10.3941/jrcr.v6i9.1144.
4. Soundararajan R, Naswa N, Sharma P, et al. SPECT-CT for characterization of extraosseous uptake of 99mTc-methylene diphosphonate on bone scintigraphy. Diagn Interv Radiol. 2013;19(5):405–410. doi:10.5152/dir.2013.054.
5. Doll R. Cancer in five continents. Proc R Soc Med. 1972;65(1):49–55.
6. Bu H, Li YL. Pathology.
7. WHO. Tumor classification and diagnostic criteria. Secondary WHO Tumor Classification and Diagnostic Criteria. Available from: <http://www.iarc.fr/en/publications/pdfs-online/pat-gen/index.php>.
8. Loutfi I, Collier BD, Mohammed AM. Nonosseous abnormalities on bone scans. J Nucl Med Technol. 2003;31(3):149–153.
9. Wale DJ, Wong KK, Savas H, Kandathil A, Piert M, Brown RK. Extraosseous findings on bone scintigraphy using fusion SPECT/CT and correlative imaging. AJR Am J Roentgenol. 2015;205(1):160–172. doi:10.2214/AJR.14.13914
10. Jacobson AF. Diffuse renal retention on bone scintigraphy in localized clear-cell renal epithelial neoplasm. J Nucl Med. 1995;36(5):817–819.
11. Wilbur MJ, Gagliardi JA, Riccio GJ, et al. Soft-tissue uptake in radionuclide musculoskeletal imaging. Appl Radiol. 1997;26(12):30–37.
12. Vallabhajosula S, Owunwanne A. Pathophysiology and mechanisms of radiopharmaceutical localization. In: Elgazzar AH, editor. The Pathophysiologic Basis of Nuclear Medicine. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006:179.
13. Zuckier LS, Freeman LM. Nonosseous, nonurologic uptake on bone scintigraphy: atlas and analysis. Semin Nucl Med. 2010;40(4):242–256. doi:10.1053/j.semnuclmed.2010.02.003
14. Sharma T, Radosevich JA, Pachori G, Mandal CC. A molecular view of pathological microcalcification in breast cancer. J Mammary Gland Biol Neoplasia. 2016;21(1–2):25–40. doi:10.1007/s10911-015-9349-9.
15. Kim MJ, Kim E-K, Kwak JY, et al. Differentiation of thyroid nodules with macrocalcifications: role of suspicious sonographic findings. J Ultrasound Med. 2008;27(8):1179–1184. doi:10.7863/jum.2008.27.8.1179
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.