Back to Journals » Journal of Pain Research » Volume 15
60-Day PNS Treatment May Improve Identification of Delayed Responders and Delayed Non-Responders to Neurostimulation for Pain Relief
Authors Naidu R , Li S , Desai MJ , Sheth S, Crosby ND , Boggs JW
Received 12 November 2021
Accepted for publication 28 February 2022
Published 14 March 2022 Volume 2022:15 Pages 733—743
DOI https://doi.org/10.2147/JPR.S349101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Krishnan Chakravarthy
Ramana Naidu,1 Sean Li,2 Mehul J Desai,3,4 Samir Sheth,5 Nathan D Crosby,6 Joseph W Boggs6
1California Orthopedics & Spine, Larkspur, CA, USA; 2Premier Pain Centers, Shrewsbury, NJ, USA; 3International Spine Pain & Performance Center, Washington, DC, USA; 4George Washington University, School of Medicine and Health Sciences, Washington, DC, USA; 5Sutter Roseville Pain Management, Roseville, CA, USA; 6SPR Therapeutics, Cleveland, OH, USA
Correspondence: Ramana Naidu, California Orthopedics & Spine, 2 Bon Air Road #120, Larkspur, CA, 94939, USA, Tel +1 608-695-7266, Email [email protected]
Objective: Conventional neurostimulation typically involves a brief (eg, ≤ 10-day) trial to assess presumed effectiveness prior to permanent implantation. Low trial conversion rates and high explant rates due to inadequate pain relief highlight the need for improved patient identification strategies. The development of a 60-day percutaneous peripheral nerve stimulation (PNS) system enables evaluation of outcomes following an extended temporary treatment period of up to 60 days, that may obviate or validate the need for permanent implant. The present study provides the first real-world evidence regarding patient response throughout a 60-day PNS treatment period.
Methods: Anonymized data listings were compiled from patients who underwent implantation of temporary percutaneous leads and opted-in to provide real-world data to the device manufacturer during routine interactions with device representatives throughout the 60-day treatment.
Results: Overall, 30% (222/747) of patients were early responders (≥ 50% pain relief throughout treatment). Another 31% (231/747) of patients initially presented as non-responders but surpassed 50% pain relief by the end of treatment. Conversely, 32% (239/747) of patients were non-responders throughout treatment. An additional 7% (55/747) of patients initially presented as responders but fell below 50% relief by the end of the treatment period.
Conclusion: An extended, 60-day PNS treatment may help identify delayed responders, providing the opportunity for sustained relief and improving access to effective PNS treatment. Compared to a conventionally short trial of ≤ 10 days, a longer 60-day PNS treatment may also help reduce explant rates by identifying delayed non-responders unlikely to benefit long-term. These scenarios support the importance of an extended 60-day temporary PNS stimulation period to help inform stepwise treatment strategies that may optimize outcomes and cost-effectiveness.
Keywords: peripheral nerve stimulation, neuromodulation, 60-day PNS, chronic pain, real-world evidence
Introduction
Chronic pain is a highly prevalent and debilitating condition affecting an estimated 18–43% of the population with total economic costs of $560-635 Billion annually across the United States.1–4 Pain frequently causes substantial disability, interference with daily activities, and leads to discouragement, anger, embitterment, and general suffering with a strong negative correlation between pain severity and health-related quality of life.5–8 In the midst of the ongoing opioid crisis in the US, peripheral nerve stimulation (PNS) has demonstrated effectiveness as a non-opioid, pain management solution for a wide range of pain conditions, including low back pain, joint pain, post-traumatic and neuropathic pain, post-operative pain, complex regional pain syndrome and oncologic pain.9–16
Current conventional neurostimulation systems for pain relief in the United States and many other regions typically involve a brief (eg, ≤7–10-day) trial to assess presumed effectiveness prior to permanent system implantation, with patients that report ≥50% pain relief typically proceeding to permanent implantation, or no trial at all.17,18 Brief trials were originally implemented to reduce the rate of failed implants and improve cost-effectiveness of therapies, yet recent reviews of conventional neurostimulation systems (including PNS, spinal cord stimulation (SCS), and dorsal root ganglion stimulation (DRGS)) have found that lack or loss of efficacy was the primary cause of up to 38–67% of system explants, with patients undergoing explant in the first three years incurring significantly higher healthcare costs.17,19–26 Additionally, prospective studies commonly report trial conversion rates >80%, but real-world conversion rates have been consistently reported to be much lower (41–65%), with patient selection, provider volume, and etiological diversity all presenting challenges to replication of clinical trial outcomes.18,27 These low trial conversion rates and high rates of explant due to inadequate pain relief suggest that improved real-world patient identification strategies are needed to better distinguish likely responders and non-responders to neurostimulation before making the decision to implant a permanent system.
The length of conventional neurostimulation trials, as a mode of identification of presumed responders and non-responders to neurostimulation, has historically been limited to ~7–10 days due to the risk of infection associated with conventional cylindrical percutaneous leads exiting the skin interface.28–31 In part because of this limitation, the predictive value of brief conventional stimulation trials is controversial,29,31–33 however single-stage implants that effectively eliminate trialing altogether fail to address the costs and risks associated with system explant in patients with inadequate pain relief, and treatment failure rates remain high when trialing is omitted.31,34 To date, little data exist regarding the potential benefits of extended (>30-day) percutaneous lead indwelling times to improve identification of responders and non-responders during temporary treatment.
In recent years, a percutaneous PNS system was developed with fine-wire, open-coil leads designed to reduce infection risk that can be safely implanted for up to 60 days.35,36 A recent review found fine-wire, coiled leads to have an estimated 60-day infection rate of 0.1% and 25-fold lower risk of infection compared to conventional cylindrical leads,30 which is similar to the infection rate in published clinical trials to-date using temporary percutaneous leads (one superficial infection at a lead exit site across 601 implanted leads, 0.17%).35,37–49 The most commonly reported adverse event has been adhesive-related skin irritation. This novel 60-day PNS treatment has demonstrated the potential to produce sustained improvements, obviating the need for a permanently implanted system altogether in some patients,16,41,50–52 while also facilitating a more detailed evaluation of the patient response throughout a longer (60-day) treatment period. The present study provides the first analysis of real-world evidence showing the evolution of pain relief in patients that previously underwent implantation of 60-day PNS leads for the treatment of pain.
Methods
Study Design and Sample
The study was a retrospective review of anonymized data listings from an existing real-world database populated with records from 6134 patients who underwent commercial implantation of temporary PNS leads (SPRINT®, SPR Therapeutics, Cleveland, OH, USA) between October 2017 and September 2021 and who gave written approval to provide real-world data to the device manufacturer. Data were stored and accessed within an ISO-27001 compliant system with appropriate data security and privacy protections including data segregation, role-based access restrictions and authentication requirements, and industry-standard data transmission encryption. The anonymized data consisted of patient reports of percent pain relief (Brief Pain Inventory – Short Form, Question #853) from routine interactions with manufacturer representatives throughout the 60-day treatment period (eg, for device support, programming, etc.). Due to the retrospective nature of the analyses and the use of anonymized data listings, IRB approval was not required.
60-Day Peripheral Nerve Stimulation Treatment
Patients in the present retrospective analysis previously underwent commercial implantation of the 60-day PNS system for various indications. The system consists of fine-wire, coiled leads implanted percutaneously, typically under ultrasound or fluoroscopic guidance. Leads are implanted remote (0.5–3 cm) from the target nerve and the externalized portion is connected to a body-mounted pulse generator that delivers the stimulation waveform (asymmetric charge-balanced biphasic pulse train, 1–30 mA, 10–200 µs, 5–150 Hz). Patients adjust stimulation intensity to maintain comfort during treatment using a wireless remote. Stimulation is delivered for up to 60 days after which the percutaneous leads are removed by clinical staff by applying gentle traction to the external portion of the lead, after which patients proceed to follow-up with their physician.
Data Analysis
Eligibility for inclusion of anonymized data in the present analysis required that the patient opted-in at the time of treatment to provide data to the device manufacturer, had completed the PNS treatment (ie, was not in treatment at the time of the analysis), provided at least one report of percent pain relief during the first two weeks of the 60-day PNS treatment, and provided at least two reports of percent pain relief overall throughout the 60-day treatment period.
Patient records extracted from the database included treatment characteristics such as nerve target, body region, number of leads implanted (ie, single vs dual lead stimulation), and duration of treatment. Pain indications were selected from a pre-populated list of the most common conditions treated with PNS and selections were non-exclusive, meaning a given patient record could indicate more than one cause of pain.
Responders were defined as those achieving ≥50% pain relief. Four response profiles were delineated: a) Early Responders: patients who reported ≥50% pain relief throughout the 60-day treatment period; b) Delayed Responders: patients who reported less than 50% pain relief within the first two weeks but ultimately achieved ≥50% pain relief before the end of the 60-day treatment period; c) Early Non-Responders: patients who never achieved ≥50% pain relief during the 60-day treatment period; and d) Delayed Non-Responders: patients who reported ≥50% pain relief within the first two weeks but ultimately fell below 50% pain relief by the end of the 60-day treatment period. The proportion of patients in each response profile was determined overall and by body region undergoing treatment (eg, low back, shoulder, knee, etc.). Mean pain relief was also summarized overall and for each response profile subgroup by week throughout the 60-day treatment period. All data are reported as mean (SD).
Results
Study Population
A total of 6134 anonymized patient records were screened, and 747 were eligible for inclusion in the present analysis (Figure 1). These 747 patient records included 4182 reports of percent pain relief throughout the 60-day PNS treatment period (5.6 ± 2.5 individual reports per patient, including end of treatment). The population represented PNS treatment in 18 different body regions and 25 different nerve targets, with low back (medial branch of the dorsal ramus), knee (femoral and/or sciatic nerves), and shoulder (axillary and/or suprascapular nerves), representing over two thirds of the population (Tables 1 and 2). The most common pain conditions treated included post-operative pain, axial back pain, osteoarthritis, and neuropathic pain (Table 3). Mean worst pain score at baseline was 8.7 ± 1.6 and mean average pain score at baseline was 6.1 ± 2.0. Sixty-one percent of patients underwent dual-lead stimulation (eg, two leads bilaterally or targeting two different nerves; 453/747) while 39% received stimulation via a single lead. Mean patient age was 62.3 ± 16.5 years and the mean duration of PNS treatment was 54.9 ± 18.2 days.
 |
Table 1 Regions of Pain Treated by 60-Day PNS |
 |
Table 2 Target Nerves for 60-Day PNS |
 |
Table 3 Pain Indications |
 |
Figure 1 Analysis flow diagram. Anonymized patient records consisting of reports of percent pain relief throughout the 60-day treatment period were assessed for inclusion in the analysis. |
Response Profiles During 60-Day PNS
Patients were categorized into four response profiles based on the evolution of their reported percent pain relief throughout the 60-day treatment period: Early Responders, Delayed Responders, Early Non-Responders, and Delayed Non-Responders. The proportions in each profile were similar across the various pain regions (Table 4).
 |
Table 4 Summary of Response Profiles |
Early Responders
Overall, 30% (222/747) of patients were classified as early responders (Table 4). Percent pain relief averaged 75.5 ± 19.7% at the time of the first report after beginning treatment, which occurred on day 4.8 ± 3.4 following lead implantation. Mean percent pain relief remained high throughout the treatment and averaged 78.4 ± 17.7% at the time of lead removal. Early responders averaged a net change of +2.8% pain relief from the first report to the end of treatment (Figure 2).
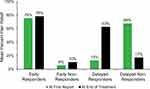 |
Figure 2 Changes in mean percent pain relief during treatment. Mean percent pain relief at the first report and the end of the 60-day treatment are shown for each response profile. |
Delayed Responders
A total of 31% (231/747) of patients were classified as delayed responders (Table 4). Percent pain relief averaged 12.9 ± 14.4% at the time of the first report after beginning treatment, which occurred on day 4.3 ± 3.6 following lead implantation. Mean percent pain relief increased week over week throughout treatment (Figure 3) and averaged 62.7 ± 26.2% at the time of lead removal. Delayed responders averaged a net change of +49.8% pain relief from the first report to the end of treatment (Figure 2). The maximum mean percent pain relief in this subgroup was achieved in the final week of the 60-day treatment period (Figure 3).
Early Non-Responders
Overall, 32% (239/747) of patients were classified as early non-responders (Table 4). Percent pain relief averaged 5.7 ± 10.8% at the time of the first report after beginning treatment, which occurred on day 4.9 ± 3.6 following lead implantation. Percent pain relief remained low throughout the treatment period in this subgroup and averaged 10.4 ± 14.3% at the time of lead removal. Early non-responders averaged a net change of +4.7% pain relief from the first report to the end of treatment (Figure 2).
Delayed Non-Responders
A total of 7% (55/747) of patients were classified as delayed non-responders (Table 4). Percent pain relief averaged 67.8 ± 18.7% at the time of the first report after beginning treatment, which occurred on day 4.1 ± 3.5 following lead implantation. Mean percent pain relief decreased week over week throughout treatment (Figure 3) and averaged 17.2 ± 17.8% at the time of lead removal. Delayed non-responders averaged a net change of −50.6% pain relief from the first report to the end of treatment (Figure 2). Mean percent pain relief in this subgroup reached its lowest level in the final two weeks of the 60-day treatment period (Figure 3).
Discussion
This study presents the first real-world evidence regarding patient response to PNS throughout a temporary, 60-day percutaneous PNS treatment period. Approximately 37% of patients were categorized as delayed responders or delayed non-responders whose initial levels of pain relief within the first two weeks of treatment were not predictive of their final response profile (Table 4). Conventional PNS systems typically employ no trial or a brief trial of ≤10 days to identify presumed responders and non-responders prior to permanent implantation. The present study suggests that categorization of patient responses to stimulation for pain relief, and the associated cost-effectiveness of neurostimulation therapies within the pain treatment algorithm, could be markedly improved by adopting significantly longer temporary stimulation treatments.
Delayed responders were one of the largest subgroups in the present study, with over 30% of patients initially reporting low pain relief (mean 12.9% pain relief in the first week) before eventually surpassing the 50% pain relief threshold and achieving their highest levels of pain relief near the end of the 60-day treatment (Figures 2 and 3). Potential factors contributing to the gradual increase in pain relief throughout the PNS treatment period include cumulative improvements in patient treatment compliance, resolution of technical issues, optimization of stimulation programming, and neurophysiological factors such as progressive reconditioning of the centrally-maintained pain state on the time course of several weeks.50,52 Clinical and real-world evidence suggest that responders to 60-day PNS treatment, including delayed responders, have the potential to achieve sustained benefits that long outlast the 60-day treatment period, obviating the need for permanently implanted conventional neurostimulation in many cases.16,40,41,50–52,54 In cases where pain returns soon after the end of the 60-day treatment, success during the temporary 60-day PNS treatment could also provide support for patients to proceed to a permanent implant. Therefore, whereas delayed responders undergoing a brief (≤10-day) treatment period may be spuriously categorized as non-responders and be forced to seek alternate therapeutic options, a 60-day treatment period may increase patient access to PNS by either providing sustained pain relief or providing evidence for the potential effectiveness of a future permanent implant.
Conversely, delayed non-responders exhibited substantial pain relief initially (mean 67.8% in the first week) before falling below 50% relief by the end of the 60-day treatment (Figures 2 and 3). Some patients may experience loss of effect due to device-related issues, and while the mean duration of treatment (54.9 days) suggests that most patients completed nearly the full 60-day PNS treatment period, analysis of the impact of early lead removal on delayed responder/non-responder rates is beyond the scope of the present analysis. Other patients may experience physiological and/or psychological accommodation or tolerance to stimulation, which has long been theorized as a cause for diminishing pain relief over time with permanent neurostimulation systems.26,55–57 Other delayed non-responders may experience waning placebo effects, which can produce false positive responses to stimulation in shorter trials and contribute to subsequent failure of permanently implanted devices.17 The potential costs and risks are significant for patients that initially present strong false positive responses during brief trials and on that basis are qualified for permanent implantation that is ultimately unlikely to be successful.25 As noted by North in an editorial on SCS trial duration, the ability to accurately predict failure holds at least as much value as predicting success in a screening protocol.58 Identifying delayed non-responders more effectively, potentially with a longer (ie, 60-day) treatment period, could therefore help avoid the physical, psychological and financial impacts associated with implantation, revision, and explant of neurostimulator systems in non-responsive patients.
In the present study, the delayed responder group did not reach its peak mean percent pain relief until the final two weeks of treatment and the delayed non-responder group similarly did not reach its minimum percent pain relief until just before the end of the 60-day treatment (Figure 2). The delayed responder and delayed non-responder groups also displayed similar mean levels of pain relief at Weeks 3 and 4, suggesting that more than 4 weeks of PNS treatment is needed to increase classification specificity (Figure 2). While much of the existing data regarding trial duration and trial outcomes are for SCS and more data are needed on trialing strategies and outcomes using conventional PNS systems, evidence indicates that a majority of PNS trials are also ≤10 days in length.11,15,28,29,32,33 For example, one text noted that, “a trial of 1–2 days is usually adequate to determine the effectiveness of PNS.”62 For SCS in particular, longer trials of up to 28 days are sometimes used outside the US, often as dictated by coverage policy, but there is no evidence that these 28-day trials are more predictive of successful permanent SCS implantation compared to shorter trials,32,33 and conventional percutaneous leads indwelling for 28 days have significantly elevated infection risk.28,30 Together, these findings suggest that temporary PNS treatment periods up to 60 days, along with percutaneous leads that can be safely implanted over this timeframe, may maximize the opportunity for identification of delayed responders and delayed non-responders.
A recent study by Eldabe et al highlighted the limitations of conventional trialing in SCS, finding that no trialing had equivalent predictive value for long-term pain control compared to conventional ≤7–10-day trials and suggesting that the clinical benefits of conventional SCS trials are limited when balanced against the infection and surgical risks of conventional systems.33 While caution should be exercised when relating experiences in SCS to current PNS technologies, its relevance to the present study warrants discussion. Notably, Eldabe et al found that more than half of positively screened patients with ≥50% pain relief during the SCS trial (mean trial duration, 9.3 days) had <50% pain relief at 3- and 6-months, suggesting a high rate of what the present study may classify as delayed non-responders whose positive trials failed to translate to effective pain relief.33 Although the study implanted patients with failed SCS trials, potentially providing an opportunity to identify delayed responders, only two of five patients with negative trials who underwent implantation were available at the 3- or 6-month follow-up, making interpretation difficult.33 Additionally, according to Eldabe et al, one of the key reasons to re-evaluate the clinical benefit of SCS trialing strategies is because the trials themselves carry significant risk of infection, surgical pain confounding patient assessments of benefit, and other significant procedural risks.33 To the extent that conventional PNS trials may carry some of the same risks, the availability of a novel percutaneous PNS system with temporary leads designed to reduce infection rates enabling indwelling periods of up to 60-days provides the opportunity to determine whether an extended PNS treatment, rather than abbreviated or absent PNS trialing, could potentially provide clinical benefit in lieu of or prior to permanent implantation. Overall, the findings of Eldabe et al, along with other studies of conventional trialing strategies, suggest that the paradigm of relatively abbreviated trialing (including no trial, acute testing, or ≤7–10-day trial) lacks specificity in general,18,32,33 reinforcing the rationale of the present study to re-evaluate the brevity of PNS treatment in an attempt to improve patient outcomes. While Eldabe et al respond to the low clinical benefit of conventional SCS trialing by challenging the necessity of SCS trials, the present study re-evaluates the brevity of PNS trials and suggests that alternate strategies like a longer temporary PNS treatment may also help improve cost effectiveness and clinical outcomes.
Limitations of the present study include its retrospective nature and the availability of data at irregular intervals across the patient population, although the large sample size in the present analysis nonetheless provides a substantial amount of longitudinal real-world data that enables detailed analysis of patient responses throughout the treatment period. Although recent studies have highlighted the importance of multidimensional analysis of patient improvement including pain, medication use, function, and health-related quality of life (QoL), the present study analyzed percent pain relief and did not have sufficient data to assess subsequent changes in function or QoL. Neuromodulation Appropriateness Consensus Committee (NACC) recommendations suggest that medication use, function, and QoL might be considered as alternate determinants of trial success with conventional stimulation, and it is possible that the early and delayed responder and non-responder rates in the present study could shift (potentially producing more early and delayed responders) with the inclusion of additional outcome dimensions.59 Additionally, as noted above much of the existing data regarding trial duration and trial outcomes are for SCS and more data are needed on trialing strategies and outcomes using conventional PNS systems. Nonetheless, NACC recommends similar trialing strategies for permanently implanted SCS, DRG, and PNS systems, and similar trends of false positive and false negative trial outcomes are seen in the PNS literature, suggesting that the trends discussed in the present study may have similarities across conventional neurostimulation modalities.59–61
Conclusions
Treatment with percutaneously implanted PNS leads for up to 60 days enabled substantial (≥50%) pain reductions in a majority (60%) of patients in a real-world setting. The responsiveness of many patients appears to evolve over the course of a 60-day PNS treatment period including both delayed improvements in pain relief for some patients and delayed loss of analgesia in others. An extended, 60-day PNS treatment may help identify delayed responders, providing the opportunity for sustained relief and improving access to effective PNS treatment. Compared to a conventionally short stimulation trial of ≤10 days, a longer 60-day PNS treatment may also help reduce explant rates by identifying delayed non-responders unlikely to benefit long-term. These scenarios support the importance of an extended 60-day temporary peripheral nerve stimulation period to help inform stepwise treatment strategies that may optimize outcomes and cost-effectiveness.
Acknowledgments
This study was supported by SPR Therapeutics.
Disclosure
R Naidu: research funding from Abbott, Boston Scientific, Nalu, Omnia Medical, and Vivex; consultant for Abbott, Avanos, Biotronik, Boston Scientific, CereVu, DoctorPlan, Exer AI, KarunaLabs, Medtronic, Nalu Medical, Omnia Medical, PainTEQ, SPR Therapeutics, and Vivex. S Li: research funding from Abbott, Avanos, Averitas Pharma, Biotronik, Boston Scientific, Nalu Medical, Nevro, PainTeq, Saluda Medical, SGX Medical, SPR Therapeutics; consultant for Avanos, Averitas Pharm, Biotronik, Boston Scientific, Nalu Medical, Nevro, PainTeq, Saluda Medical, Scilex Pharma, SPR Therapeutic, and Vertos; stock ownership/options in Nalu Medical, and National Spine and Pain Centers. MJ Desai: research funding from Abbott, Amgen, Bioness, Mainstay, Nalu, Nature Cell, Nevro, Seikagaku, SPR Therapeutics, and Vivex; consultant for Abbott, Avanos and Relievant; Stock ownership/options in SPR Therapeutics, SynerFuse, and Virdio. S Sheth: consultant for Nevro, Medtronic, Boston Scientific; speaker’s bureau for Medtronic; physician advisory board for Medtronic, Boston Scientific. ND Crosby: employee of SPR Therapeutics; stock ownership/options in SPR Therapeutics. JW Boggs: employee of SPR Therapeutics; stock ownership/options in SPR Therapeutics. He also has multiple patents owned by and issued to SPR Therapeutics. The authors report no other conflicts of interest in this work.
References
1. Henschke N, Kamper SJ, Maher SG. The epidemiology and economic consequences of pain. Mayo Clin Proc. 2015;90(1):139–147. doi:10.1016/j.mayocp.2014.09.010
2. Kuehn B. Chronic pain prevalence. JAMA. 2018;320(16):1632.
3. Pitcher MH, Von Korff M, Bushnell MC, et al. Prevalence and profile of high-impact chronic pain in the United States. J Pain. 2019;20(2):146–160. doi:10.1016/j.jpain.2018.07.006
4. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi:10.15585/mmwr.mm6736a2
5. Gore M, Brandenburg NA, Dukes E, et al. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005;30(4):374–385. doi:10.1016/j.jpainsymman.2005.04.009
6. Gormsen L, Rosenberg R, Bach FW, et al. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur J Pain. 2010;14(2):
7. Haythornthwaite JA, Benrud-Larson LM. Psychological aspects of neuropathic pain. Clin J Pain. 2000;16(2 Suppl):S101–S105. doi:10.1097/00002508-200006001-00017
8. Ciaramitaro P, Mondelli M, Logullo F, et al. Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst. 2010;15(2):120–127. doi:10.1111/j.1529-8027.2010.00260.x
9. Hassenbusch SJ, Stanton-Hicks M, Schoppa D, et al. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996;84(3):415. doi:10.3171/jns.1996.84.3.0415
10. Eisenberg E, Waisbrod H, Gerbershagen HU. Long-term peripheral nerve stimulation for painful nerve injuries. Clin J Pain. 2004;20(3):143–146. doi:10.1097/00002508-200405000-00003
11. Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci. 2007;14(3):216–221. doi:10.1016/j.jocn.2005.11.007
12. Deer TR, Esposito MF, McRoberts WP, et al. A systematic literature review of peripheral nerve stimulation therapies for the treatment of pain. Pain Med. 2020;21(8):1590–1603. doi:10.1093/pm/pnaa030
13. Deer TR, Naidu R, Strand N, et al. A review of the bioelectronic implications of stimulation of the peripheral nervous system for chronic pain conditions. Bioelectron Med. 2020;6(1):9. doi:10.1186/s42234-020-00045-5
14. Xu J, Sun Z, Wu J, et al. Peripheral nerve stimulation in pain management: a systematic review. Pain Physician. 2021;24(2):E131–E152.
15. Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics. 2008;5(1):100–106. doi:10.1016/j.nurt.2007.11.005
16. Mainkar O, Solla CA, Chen G, et al. Pilot study in temporary peripheral nerve stimulation in oncologic pain. Neuromodulation. 2020;23(6):819–826. doi:10.1111/ner.13139
17. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation. 2017;20(6):543–552. doi:10.1111/ner.12634
18. Murphy KR, Han JL, Hussaini SMQ, et al. The volume‐outcome effect: impact on trial‐to‐permanent conversion rates in spinal cord stimulation. Neuromodulation. 2017;20(3):256–262. doi:10.1111/ner.12526
19. Van Buyten JP, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642–649. doi:10.1111/ner.12642
20. Hayek SM, Veizi E, Hanes M. Treatment‐limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603–609. doi:10.1111/ner.12312
21. Dupré DA, Tomycz N, Whiting D, et al. Spinal cord stimulator explantation: motives for removal of surgically placed paddle systems. Pain Pract. 2018;18(4):500–504. doi:10.1111/papr.12639
22. McJunkin TL, Lynch PJ, Srejic E. Complications of peripheral nerve stimulation: open technique, percutaneous technique, and peripheral nerve field stimulation. In: Ranson M, Pope J, Deer T, editors. Reducing Risks and Complications of Interventional Pain Procedures. Philadelphia: Elsevier Saunders; 2012.
23. Weigel R, Capelle -H-H, Krauss JK. Failure of long-term nerve root stimulation to improve neuropathic pain. J Neurosurg. 2008;108(5):921–925. doi:10.3171/JNS/2008/108/5/0921
24. Kumar K, Wilson J. Factors affecting spinal cord stimulation outcome in chronic benign pain with suggestions to improve success rate. In: Sakas DE, Simpson BA, Krames ES, editors. Operative Neuromodulation. Springer; 2007:91–99.
25. Han JL, Murphy KR, Hussaini SMQ, et al. Explantation rates and healthcare resource utilization in spinal cord stimulation. Neuromodulation. 2017;20(4):331–339. doi:10.1111/ner.12567
26. Reddy RD, Moheimani R, Yu GG, et al. A review of clinical data on salvage therapy in spinal cord stimulation. Neuromodulation. 2020;23(5):562–571. doi:10.1111/ner.13067
27. Huang KT, Martin J, Marky A, et al. A national survey of spinal cord stimulation trial‐to‐permanent conversion rates. Neuromodulation. 2015;18(2):133–140. doi:10.1111/ner.12199
28. North R, Desai MJ, Vangeneugden J, et al. Postoperative infections associated with prolonged spinal cord stimulation trial duration (PROMISE RCT). Neuromodulation. 2020;23(5):620–625. doi:10.1111/ner.13141
29. Chincholkar M, Eldabe S, Strachan R, et al. Prospective analysis of the trial period for spinal cord stimulation treatment for chronic pain. Neuromodulation. 2011;14(6):523–529. doi:10.1111/j.1525-1403.2011.00384.x
30. Ilfeld BM, Gabriel RA, Saulino MF, et al. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract. 2017;17(6):753–762. doi:10.1111/papr.12523
31. North RB, Calodney A, Bolash R, et al. Redefining spinal cord stimulation “trials”: a randomized controlled trial using single‐stage wireless permanent implantable devices. Neuromodulation. 2020;23(1):96–101. doi:10.1111/ner.12970
32. Weinand ME, Madhusudan H, Davis B, et al. Acute vs. prolonged screening for spinal cord stimulation in chronic pain. Neuromodulation. 2003;6(1):15–19. doi:10.1046/j.1525-1403.2003.03002.x
33. Eldabe S, Duarte RV, Gulve A, et al. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost-effectiveness (TRIAL-STIM)? A randomised controlled trial. Pain. 2020;161(12):2820. doi:10.1097/j.pain.0000000000001977
34. Gilligan C, Volschenk W, Russo M, et al. An implantable restorative-neurostimulator for refractory mechanical chronic low back pain: a randomized sham-controlled clinical trial. PAIN. 2021. doi:10.1097/j.pain.0000000000002258
35. Rauck RL, Cohen SP, Gilmore CA, et al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation. 2014;17(2):188–197. doi:10.1111/ner.12102
36. Ilfeld BM, Grant SA, Gilmore CA, et al. Neurostimulation for postsurgical analgesia: a novel system enabling ultrasound-guided percutaneous peripheral nerve stimulation. Pain Pract. 2017;17(7):892–901. doi:10.1111/papr.12539
37. Chae J, Wilson RD, Bennett ME, et al. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case series. Pain Pract. 2013;13(1):59–67. doi:10.1111/j.1533-2500.2012.00541.x
38. Chae J, Yu DT, Walker ME, et al. Intramuscular electrical stimulation for hemiplegic shoulder pain: a 12-month follow-up of a multiple-center, randomized clinical trial. Am J Phys Med Rehabil. 2005;84(11):832–842. doi:10.1097/01.phm.0000184154.01880.72
39. Deer TR, Gilmore CA, Desai MJ, et al. Percutaneous peripheral nerve stimulation of the medial branch nerves for the treatment of chronic axial back pain in patients after radiofrequency ablation. Pain Med. 2021;22(3):548–560. doi:10.1093/pm/pnaa432
40. Gilmore C, Ilfeld B, Rosenow J, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: a multicenter, randomized, placebo-controlled trial. Reg Anesth Pain Med. 2019;44(6):637–645. doi:10.1136/rapm-2018-100109
41. Gilmore CA, Desai MJ, Hopkins TJ, et al. Treatment of chronic axial back pain with 60-day percutaneous medial branch PNS: primary endpoint results from a prospective, multicenter study. Pain Pract. 2021;21:877–889. doi:10.1111/papr.13055
42. Gilmore CA, Kapural L, McGee MJ, et al. Percutaneous peripheral nerve stimulation (PNS) for the treatment of chronic low back pain provides sustained relief. Neuromodulation. 2019;22(5):615–620. doi:10.1111/ner.12854
43. Ilfeld BM, Ball ST, Gabriel RA, et al. A feasibility study of percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty. Neuromodulation. 2019;22(5):653–660. doi:10.1111/ner.12790
44. Ilfeld BM, Gilmore CA, Grant SA, et al. Ultrasound-guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: a prospective feasibility study. J Orthop Surg Res. 2017;12(1):4. doi:10.1186/s13018-016-0506-7
45. Renzenbrink GJ, Ijzerman MJ. Percutaneous neuromuscular electrical stimulation (P-NMES) for treating shoulder pain in chronic hemiplegia. Effects on shoulder pain and quality of life. Clin Rehabil. 2004;18(4):359–365. doi:10.1191/0269215504cr759oa
46. Wilson RD, Bennett ME, Nguyen VQC, et al. Fully implantable peripheral nerve stimulation for hemiplegic shoulder pain: a multi-site case series with two-year follow-up. Neuromodulation. 2018;21(3):290–295. doi:10.1111/ner.12726
47. Wilson RD, Gunzler DD, Bennett ME, et al. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93(1):17–28. doi:10.1097/PHM.0000000000000011
48. Wilson RD, Harris MA, Gunzler DD, et al. Percutaneous peripheral nerve stimulation for chronic pain in subacromial impingement syndrome: a case series. Neuromodulation. 2014;17(8):
49. Yu DT, Chae J, Walker ME, et al. Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: a pilot study. Arch Phys Med Rehabil. 2001;82(1):20–25. doi:10.1053/apmr.2001.18666
50. Gilmore CA, Ilfeld BM, Rosenow JM, et al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2020;45(1):44–51. doi:10.1136/rapm-2019-100937
51. Gilmore CA, Kapural L, McGee MJ, et al. Percutaneous peripheral nerve stimulation for chronic low back pain: prospective case series with 1 year of sustained relief following short-term implant. Pain Pract. 2020;20(3):310–320. doi:10.1111/papr.12856
52. Deer TR, Eldabe S, Falowski SM, et al. Peripherally induced reconditioning of the central nervous system: a proposed mechanistic theory for sustained relief of chronic pain with percutaneous peripheral nerve stimulation. J Pain Res. 2021;14:721–736. doi:10.2147/JPR.S297091
53. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23(2):129–138.
54. Pingree MJ. Real World Evidence of Sustained Improvements Following Percutaneous PNS: A Retrospective Cross-Sectional Follow-Up Survey of 354 Patients. Miami, FL: American Society of Pain & Neuroscience; 2021.
55. Doleys DM. Psychological factors in spinal cord stimulation therapy: brief review and discussion. Neurosurg Focus. 2006;21(6):1–6. doi:10.3171/foc.2006.21.6.4
56. Huygen FJ, Liem L, Nijhuis H, et al. Evaluating dorsal root ganglion stimulation in a prospective Dutch cohort. Neuromodulation. 2019;22(1):80–86. doi:10.1111/ner.12798
57. Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58(3):481–496. doi:10.1227/01.NEU.0000192162.99567.96
58. North RB. SCS trial duration. Neuromodulation. 2003;6(1):4–5. doi:10.1046/j.1525-1403.2003.03010.x
59. Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17(6):515–550.
60. Slavin KV, Colpan ME, Munawar N, et al. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: a single-institution experience and review of the literature. Neurosurg Focus. 2006;21(6):1–5. doi:10.3171/foc.2006.21.6.8
61. Trentman TL, Zimmerman RS, Dodick DW. Occipital nerve stimulation: technical and surgical aspects of implantation. In: Slavin KV, editor. Peripheral Nerve Stimulation. Basel, Switzerland: Karger; 2011:96–108.
62. Stanton-Hicks, M. Peripheral nerve stimulation for pain peripheral neuralgia and complex regional pain syndrome. In: Krames ES, Peckham PH, Rezai AR, editor. Neuromodulation. New York: Elsevier;2009:397–407.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

