Back to Journals » Cancer Management and Research » Volume 13
5-Fu-Based Doublet Regimen in Patients Receiving Perioperative or Postoperative Chemotherapy for Locally Advanced Gastric Cancer: When to Start and How Long Should the Regimen Last?
Authors Liu Z , Wang Y, Shan F , Ying X, Zhang Y, Li S, Jia Y, Li Z , Ji J
Received 7 October 2020
Accepted for publication 25 November 2020
Published 11 January 2021 Volume 2021:13 Pages 147—161
DOI https://doi.org/10.2147/CMAR.S285361
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Zining Liu,* Yinkui Wang,* Fei Shan, Xiangji Ying, Yan Zhang, Shuangxi Li, Yongning Jia, Ziyu Li, Jiafu Ji
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Gastrointestinal Cancer Center, Peking University Cancer Hospital & Institute, Beijing 100142, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ziyu Li
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Gastrointestinal Cancer Center, Peking University Cancer Hospital & Institute, Beijing 100142, People’s Republic of China
Email [email protected]
Background: The duration and the optimal time to adjuvant chemotherapy (TAC) in locally advanced gastric cancer (LAGC) have net not been sufficiently demonstrated. Sequential adjuvant chemotherapy (AC) after neoadjuvant chemotherapy plus gastrectomy is increasingly utilized, making the question more complicated.
Patients and Methods: Data were collected from patients with LAGC who underwent 5-Fu-based doublet regimens as adjuvant treatment after gastrectomy in a single-center database. TAC and duration (cycles) were used to evaluate survival outcomes.
Results: A total of 816 patients were included. Patients received over six cycles and TAC less than 42 days significantly correlated with better survival (log-rank Ptrend< 0.001). The analysis of TAC and number cycles were separately applied in perioperative chemotherapy (PEC) and postoperative chemotherapy (POC) group using Cox regression. The number of cycles revealed a statistical significance improving OS rate both in POC (HR=0.904, 95% CI=0.836– 0.977, P=0.011) and PEC (HR=0.887, 95% CI=0.798– 0.986, P=0.026), while only in POC did the TAC show an increasing trend of risk with borderline significance (OS: HR=1.008, 95% CI=0.999– 1.018, P=0.094; PFS: HR=1.009, 95% CI=1.000– 1.018, P=0.055). A spline model demonstrates the less improvement in survival after cycles of chemotherapy reaching six.
Conclusion: Our findings suggest that TAC is more likely to downregulate the survival benefit in POC rather than PEC, while overall survival is susceptible to cumulative cycles of chemotherapy in both groups. Furthermore, six cycles of chemotherapy tended to reach the maximum survival benefits. Prospective confirmation is required.
Keywords: gastric cancer, chemotherapy, time to initiation, duration, restricted cubic spline
Introduction
Gastric cancer (GC) is the fifth most commonly diagnosed neoplasm, accounting for 5.7% of all cancers worldwide; around 50% of GC cases occur in China.1,2 Unlike Japan, South Korea, and several other East Asian countries, most patients with GC in China are diagnosed at an already advanced stage with a high tumor load; thus, radical gastrectomy is the only available curative treatment. However, evidence indicates it has limited efficacy.
Relative to surgery-only treatment, many large-scale prospective randomized controlled studies have demonstrated improvements in overall survival (OS) and progression-free survival (PFS) in patients treated with 5-fluorouracil (5-Fu)-based perioperative chemotherapy (PEC) or postoperative chemotherapy (POC) alone.3–7 In order to eradicate micrometastases and alleviate tumor load, chemotherapy of six cycles or more is required.5,7 Moreover, the time to adjuvant chemotherapy (TAC) should be within 6–8 weeks, as indicated by several clinical trials.3,5,7
However, these treatment protocol blueprints are overshadowed by poor completion rates, and the restricted time frame for treatment is not easy to adhere to for patients with poor health status or more severe postoperative complications.8,9 While many studies have examined the survival outcomes in POC patients with delayed TAC, the findings are inconsistent.10–12 On the other hand, there have been a few studies of PEC outcomes with delayed TAC.13 Moreover, little is known about the “flexion point” in the duration of chemotherapy; that is, the time frame in which optimal treatment efficacy can be achieved. Most retrospective studies have focused on treatment completion rather than the treatment sequence or strategies.14 There is also much concern as to whether TAC and duration have the same influences in PEC and POC patients.
Therefore, in the present study, we sought to explore the influences of TAC and the number of chemotherapy cycles on patient survival. This study was conducted among a sample of LAGC patients who received PEC or POC.
Patients and Methods
Patients
The data were obtained from a retrospective database of all patients receiving adjuvant chemotherapy after curative gastrectomy at the Peking University Cancer Hospital and Institute from January 1, 2007, to January 1, 2018.
The inclusion criteria included: 1) A proven diagnosis of gastric adenocarcinoma by preoperative (for pathological complete response only) and postoperative pathology; 2) complete clinicopathological data recorded; 3) no signs of distant metastasis at first visit; 4) patients had undergone adjuvant chemotherapy after surgery; and 5) curative gastrectomy was performed.
The exclusion criteria were as follows: 1) Lack of data on chemotherapy initiation date or number of cycles of chemotherapy; 2) patients treated with mono-, triple-drug therapy, or dual drugs without a 5-Fu-based regimen during adjuvant treatment; 3) patients who received radiotherapy or targeted therapy before relapse; 4) patients who received intraperitoneal chemotherapy or hyperthermia intraperitoneal chemotherapy; 5) patients with R1/R2 resection or suspected of having metastasis during the initiation of adjuvant chemotherapy after curative resection; 6) patients with D0/D1/D1+ lymphadenectomy; 7) patients at stage T1a-bN0 without preoperative chemotherapy; and 8) prior history of gastrointestinal tumor (Figure 1). In total, 816 eligible patients were identified in the retrospective database.
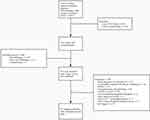 |
Figure 1 Selection of patients for inclusion. |
Regimen and Measurement of Cycles and TAC
There are seven types of 5-Fu-based dual-chemotherapy regimen protocols, including SOX (oxaliplatin plus S-1), CapeOX (oxaliplatin plus capecitabine), FOLFOX (oxaliplatin plus 5-Fu/4-Lv), CS (cisplatin plus S-1), PX (paclitaxel plus capecitabine), PS (paclitaxel plus S-1), and XELIRI (irinotecan plus capecitabine). The treatment protocols administered in this study were in accordance with the guidelines of the NCCN15 and are described in Supplementary Table S1. In PEC, theoretically, postoperative chemotherapy regimen consisted of the same chemotherapy regimen as prescribed preoperatively. A small fraction of NACT patients received altered 5-Fu-based chemotherapy regimens after gastrectomy (eg, from SOX to PS). In this case, the adjuvant chemotherapy regimen was regarded as the “major regimen” and must be within the dual drug selection. For patients who received NACT, the number of cycles was the sum of the perioperative cycles, while for patients who received only adjuvant chemotherapy, the number of cycles was the number of postoperative cycles. To assess the influence of the number cycles, the three 14-day cycles of FOLFOX were calculated as two 21-day cycles, consistent with the other 5-Fu-based regimens. The time to adjuvant chemotherapy was defined as the number of days from the completion of radical surgery to the first administration of chemotherapy. Patients were stratified into four groups based on the number of cycles completed and TAC: A) Patients who received more than six cycles of chemotherapy and TAC ≤42 days (n=365); B) patients who received more than six cycles of chemotherapy with TAC >42 days (n=137); C) patients who received less than six cycles of chemotherapy with TAC >42 days (n=192); and D) patients who received less than six cycles of chemotherapy with TAC >42 days (n=123).
Data Collection
The patient characteristics, including age, body mass index (BMI), gender, American Society of Anesthesiologists score (ASA), ECOG performance status, comorbidities, tumor location, tumor diameter (on short axis), differentiation grade, vascular involvement, pathological (p) or post-therapy pathological (yp) TNM stage, type of resection, complications, adverse events, total cycles of chemotherapy, date of NACT initiation, date of surgery, date of adjuvant chemotherapy initiation, date of progression or recurrence, OS, and PFS, have been previously described.16 For POC patients, OS or PFS was defined as time from the beginning of radical resection until confirmation of disease progression or death. For PEC patients, OS or PFS was defined as time from the start of chemotherapy until confirmation of disease progression or death. Postoperative complications were classified according to the Clavien-Dindo classification system. Adverse events were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.17 The clinical and pathological data, measure of response for NACT patients, and ways of follow-up were described in our earlier study.16
Statistical Analysis
Continuous variables were summarized as mean±standard deviation or median (IQR) and were compared across groups using the Kruskal–Wallis test. Categorical variables were analyzed using the Chi-squared test. The relationships between clinical and pathological factors and long-term PFS and OS were assessed using univariate Log rank tests and a multivariate Cox proportional hazard model. Tumor or treatment characteristics that achieved a P-value<0.10 in univariate analysis were included in the multivariate analysis. A correlation matrix was used to examine parameters with high collinearity. Correlation coefficients of >0.4 were considered as showing a positive correlation between variables. Continuous covariates were dichotomized or categorized if necessary to reduce multicollinearity. To model the nonlinear relationship between TTS and OS (or PFS), a restricted cubic spline (RCS) procedure was fitted with three internal knots (10th, 50th, and 90th centiles as suggested by Harrell).18 Testing for trends can be applied based on various statistical hypothesis when necessary. For all analyses, P<0.05 was considered statistically significant. Statistical analyses were performed using SE STATA (Stata Statistical Software, release 15.1; Stata Corp, College Station, TX, USA).
Results
Patient Characteristics
The baseline clinicopathological characteristics of the patients are summarized in Table 1. Significant differences were found in age, tumor location, resection type, neoadjuvant chemotherapy, grades of complications, and adverse events. Patients receiving adjuvant chemotherapy greater than 42 days and less than six cycles were more likely to be older, have diffused location tumors, and have undergone total gastrectomy. In addition, patients who suffered from higher grade postoperative complications were more susceptible to time delay, while patients who received sufficient cycles tended to have a higher grade of adverse events. The distribution of TAC and chemotherapy cycles is shown in Figure 2 with a median time to adjuvant chemotherapy of 36 days (IQR=31–45 days) and a median of six cycles of chemotherapy (IQR=4–8 cycles).
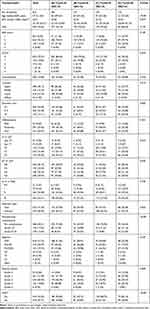 |
Table 1 Patient Characteristics Categorized by Time to Initiation and Duration of Chemotherapy |
Follow-Up Survival and Recurrence
Among the entire cohort, there were 264 deaths and 325 recurrences. The median follow-up period among all patients was 49 months (IQR=29–75 months). Kaplan–Meier curves for OS and PFS are presented in Figure 3 with statistically significant differences and statistical trends between the previously stratified groups evident in OS (P=0.008 and Ptrend<0.001 from group A to D) and marginally significant differences and significant trends in PFS (P=0.050 and Ptrend=0.006 from group A to D). The Kaplan–Meier curves for duration or for initiation are, shown in Supplementary Figure S1 and S2, respectively.
 |
Figure 3 (A) Kaplan–Meier estimates for overall survival by group. (B) Kaplan–Meier estimates for progression-free survival by group. |
In the univariate analyses, BMI, ASA, ECOG, comorbidities, tumor location, diameter, differentiation, postoperative complications, lymphovascular invasion, p/yp T/N/TNM stage, type of resection, regimen, adverse event, cycles <6 (or as continuous variables), and TAC ≥42 days (or as continuous variables) were related to poorer survival prognosis (P<0.10) (Table 2). Given that both ECOG and ASA measure similar things, ECOG was included in the multivariate model rather than ASA. Because of collinearity, lymphovascular invasion (r=0.49, with p/yp N stage), type of gastrectomy (r=−0.47, with tumor location), and (p/yp) T and (p/yp) N stages were integrated as (p/yp) TNM stage. After adjusting for potential confounders in the multivariate Cox regression model, more than six cycles of chemotherapy was associated with a significant improvement in OS (HR=1.335, 95% CI=1.040–1.713, P=0.023), but not significant in PFS (HR=1.201, 95% CI=0.958–1.505, P=0.113). However, for TAC >42 days, there were no statistically significant differences in the survival benefit in terms of OS and PFS (Table 2). To further demonstrate the relationships between covariates and survival outcomes, the number of cycles and TAC were considered as continuous predictive variables in a Cox model of proportional risks (Model 2). In the second model, the results were almost the same as the dichotomized model: cycles (as a continuous variable) was a significant predictor only of OS (HR=0.930, 95% CI=0.878–0.985, P=0.014). This suggests a trend whereby a greater number of cycles is associated with a decreased risk of death. Again, no such relationship was observed for TAC (Table 3).
 |
Table 2 Univariate and Multivariate Analyses of Prognostic Factors |
 |
Table 3 Multivariate Cox Hazards Regression Model for the Predictable Risk of OS and PFS in Different Covariates Inclusion in Whole Patients |
Effect of PEC Vs POC on Chemotherapy Initiation and Duration
Given that the TNM stage and response to chemotherapy are not equivalent in patients with NACT treatment versus those without, a subsequent analysis was performed to investigate the influence of these covariates on the PEC and POC groups, respectively (Tables 4 and 5). Models 3 and 4 demonstrate the initiation/duration effect on POC patients. When the variables were dichotomized, both cycles <6 (HR=1.372, 95% CI=0.957–1.967, P=0.085) and TAC >42 days (HR=1.415, 95% CI=0.964–2.077, P=0.076) were close to being statistically significant predictors of OS but not PFS (Model 3). However, in the continuous Cox regression model (Model 4), a significant downward association was detected between the cumulative number of cycles and OS risk (HR=0.904, 95% CI=0.836–0.977, P=0.011); this relationship was approaching significance for PFS (HR=0.942, 95% CI=0.880–1.008, P=0.086). TAC also showed a reliable trend towards increased OS (HR=1.008, 95% CI=0.999–1.018, P=0.094), and an almost significant tendency for PFS (HR=1.009, 95% CI=1.000–1.018, P=0.055).
 |
Table 4 Multivariate Cox Hazards Regression Model for the Predictable Risk of OS and PFS in Different Covariate Inclusion in POC Patients |
 |
Table 5 Multivariate Cox Hazards Regression Model for the Predictable Risk of OS and PFS in Different Covariate Inclusion in PEC Patients |
In the PEC subgroup, TAC, both the dichotomous and continuous variables were not significantly associated with an OS/PFS benefit (Model 5–6). The number of cycles again was associated with significant improvement in OS, for both the dichotomized (HR=1.567, 95% CI=1.077–2.282, P=0.019, Model 5) and continuous variable (HR=0.887, 95% CI=0.798–0.986, P=0.026, Model 6), but was not a significant predictor of reduced PFS (P=0.120, Model 5; P=0.140, Model 6).
Interpretation of the Nonlinear Relationship Based on Cox Regression
Above, we used Cox models to demonstrate significant or reliable associations between initiation time/chemotherapy duration and OS/PFS risk. To fully explain the relationships between these variables and survival risks, restricted cubic-spline Cox proportional hazard regressions were performed. Since the number of cycles was associated with survival outcomes of both PEC and POC patients, the analysis of cycles was based on the whole sample of patients. TAC, as reported above, was not associated with survival outcome in the PEC group; thus, it was only entered as a predictor for the POC patients. For the analysis of duration, univariate cubic splines were first performed. The nonlinear Wald test result was 0.042 for OS and 0.014 for PFS. The univariate RCS revealed that patients who received six cycles of chemotherapy had the lowest survival risks (Figure 4A and B), whilst the steep downward trend turned smooth when the cycle number reached after five and the flexion point was approximately near the six. When adjusted for covariates in Model 2, the Wald test failed to identify a significant non-linear model over a linear model with P-values of 0.342 for OS and 0.164 for PFS. Thus, the regression spline was only applied in the univariate model, which suggested that six cycles tended to achieve maximum survival benefit.
Adverse Events
The major adverse events during chemotherapy are presented in Table 6. No deaths related to chemotherapy were observed. There is a significant trend for higher grade of leucopenia, thrombocytopenia, hepatotoxicity, neurotoxicity, skin reaction, and fatigue in patients who completed the cycles (Ptrend<0.05). Compared with POC, PEC patients with sufficient cycles (≥6) had a higher chance to undergo thrombocytopenia (Ptrend=0.003) but a lower fatigue rate (Ptrend=0.031). Except from this, the toxicities between PEC and POC were comparative.
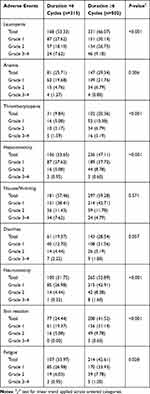 |
Table 6 Comparison of Treatment-Related Adverse Events Between the Completion and Incompletion Groups |
Discussion
Although the role of chemotherapy remains controversial in resectable GC, its benefit has been demonstrated in several large clinical trials.19 Cirera et al20 provided the first evidence of the positive effects of adjuvant chemotherapy for GC patients. In 2006, the MAGIC trial provided the first evidence of the promising effects of PEC in improving survival rates of patients with resectable adenocarcinoma of the esophagus or GEJ.5 While these treatment modalities may serve different purposes, one of their primary common objectives is to eliminate subclinical micrometastasis and prevent recurrence.21,22 Both the duration and the timing of chemotherapy initiation are important. Currently, the “optimal” initiation time and duration of chemotherapy are based on the protocols used in clinical trials; to date, neither of these standards have been systematically studied.
In the present study, we used stringent inclusion/exclusion criteria to extract data from the retrospective database at our center. Patients received either perioperative or postoperative chemotherapy alone, but the eligible regimens were restricted to dual drug regimens that were 5-Fu based. The 5-Fu based doublet treatment, eg, with a oxaliplatin cisplatin or paclitaxel analog, has demonstrated some survival benefit for patients with resectable GC or GEJ, and is widely adopted across China.23–25 Although 5-Fu has been the backbone of treatment for LAGC for decades, the toxicity, tolerance, completion rate, and prognosis differ among mono, dual, and triplet therapies.26,27 Currently, 5-Fu based combinations are widely adopted for advanced gastric cancer in Asia and recently in European countries. The evidence is based on the results in several prospective studies that a longer survival is observed in the doublet group compared with S-1 alone in AGC or end-staged patients.28,29 Doublet therapy, contrarily, could lead to higher incidence of adverse events and may require dose reduction. In the SPIRITS trial, more grade 3–4 adverse events were observed in patients assigned to S-1 plus cisplatin than among those assigned to S-1 alone.30 Controlling for these basic factors allowed us to further study the optimal timing and duration of chemotherapy. According to our results, more than six cycles of chemotherapy and the initiation of chemotherapy before 6 weeks were associated with greater survival in patients who received either perioperative or postoperative chemotherapy. However, the results revealed differing sensitivity to initiation time between perioperative and postoperative chemotherapy. Specifically, only patients who did not receive preoperative chemotherapy were likely to be susceptible to the time of adjuvant chemotherapy initiation, while all patients exhibited survival advantages with an increasing number of cycles of chemotherapy. The multivariate results were interpreted with the help of nonlinear tests and spline models to avoid data-driven categorization.31
Although many studies have examined the impact of timing of chemotherapy after gastrectomy,10–12 there are limited studies of patients who have received PEC.13 To our knowledge, this is the first study that has attempted to evaluate the intrinsic similarities and differences between these treatment modalities. Previous studies have given much attention to the survival benefit of early initiation of TAC in patients who received gastrectomy directly after diagnosis.32,33 However, as preoperative treatment is increasingly utilized and has been included in the NCCN guidelines for years,34 the evidence acquired in relation to POC should be cautiously applied to PEC. In preoperative treatment, there is theoretically less tumor burden and micrometastatic foci outside the surgical field after radical surgery. Thus, the window of postoperative chemotherapy might be widened in PEC patients. Brenkman et al13 first examined the effect of timing of chemotherapy among PEC patients with GC and found no improvement in the early initiation group (<6 weeks). In our study, to elucidate the relationship with prognosis, TAC was entered into the spline Cox model as a continuous variable. The linear trend observed between the log HR and TAC was of borderline significance (P=0.094 in OS and P=0.055 in PFS). Similar to Brenkman et al, the relationships between TAC and survival outcomes in the POC group were out-of-order. The results confirmed our “widened window” hypothesis, indicating that PEC patients are more tolerant of a long waiting period before chemotherapy initiation and could benefit from the additional time to recover physically after surgery or related complications.35
There is no defined standard stipulating the number of cycles of POC or PEC that confer maximum survival benefit or cost-effectiveness. The recommended duration is based on the protocols of previous clinical trials, and is commonly cited as six cycles or more.3,5,6,36 Rather than simply focusing on whether or not the chemotherapy protocol was completed, several retrospective studies have investigated patient survival benefits in relation to the accumulation of POC cycles.9,14,37 Xiao et al9 examined a cohort of 1,288 patients who were stratified into five groups according to the number of postoperative cycles they received. The results indicated that patients who received six-to-seven cycles had similar cancer-specific survival to those who received eight cycles or more. As for PEC, there are few similar studies. In the MRC OE05 trial, patients who received two cycles of CF preoperative chemotherapy showed comparable survival outcomes to patients who received four cycles of ECX, but this study did not examine POC.38 Moreover, in spite of the proven benefit of PEC, there is still debate as to the role of POC.39 In the MIROX trial of colorectal cancer, patients who received PEC had comparable survival outcomes to those who received POC; all patients received a total of 12 cycles of chemotherapy treatment.40 In the current study, to elucidate the number of cycles and prognostic outcomes, patients with PEC and POC were separately analyzed using multivariate models. The number of cycles showed a statistical trend in both groups (OS: P=0.009 in POC, P=0.036 in PEC). Although this does not mean that the accumulation of cycles influences patient survival in the same way, this result indicates that both PEC and POC should be susceptible to increased numbers of chemotherapy cycles.
Utilizing the nonlinear regression technique, we found a nonlinear relationship between the number of cycles and log HR in the univariate Cox model. Although the downward trend towards survival risk was sharp before the first five cycles, it turned smooth afterward and gradually minimized when the number of cycles reached six. This is partially in accordance with previous retrospective studies of the survival benefit of shorter-term use of chemotherapy.14,41 Although Cox regression did not fit the nonlinear hypothesis, in further analysis after adjustment for covariates, we suggest that the multivariate model only reflects an ideal circumstance when all patients’ clinical conditions are thought to be equal. Because of the poor chemotherapy completion rate reported in previous studies, the real-world is mixtures in which the situation is always more complicated.8 It is reasonable to assume that patients with a longer duration of chemotherapy may have potentially longer OS and PFS, but the improvement rate in tumor response and the patient’s quality-of-life may reduce as dosage and toxicity accumulate. In our result the cumulation of cycles strongly correlated with incidence of higher grade of leucopenia (r=0.574, P<0.001, data not shown); this may lower the cost-effectiveness for patients and their families.9,–41–44 Our result demonstrates a potentially small survival benefit of more than six cycles of chemotherapy in patients with PEC and POC; this warrants further investigations.
Our study has several limitations. First, this was a retrospective study conducted in a single center and reasons for delaying or quitting chemotherapy were not systematically recorded. Second, we arbitrarily merged PEC and POC as a whole group in some of the analysis; there is no clinical evidence to support this approach. Third, although the 8th ypTNM staging system was consistent with patients who underwent NACT, the degree of prognosis at each stage may vary from that of the traditional pTNM stage.16,45,46 Fourth, treatment after recurrence was not specified in the present study that might become a confounder for patients OS. Though PFS rate is significantly higher in patients with earlier initiation plus longer duration in our initial analysis, it fails to show a significant difference when tested separately. Lastly, despite detailed records of adverse events, dose reductions are not reflected in this study, which is another crucial factor in patient prognosis. In fact, we do find the difference in completion rate between PEC and POC patients (70.78% vs 53.50%, P<0.001). We value this result as it might indicate: 1) Since so many studies suggested improved survival outcome in patients with sufficient duration, a higher completion rate in PEC might shed light on the benefit of NACT and a need to investigate the optimal modality for perioperative chemotherapy;47 2) There is a potential advantage in patients with PEC who are more likely to receive minimal invasive procedure which results in lower overall complications and hospital stay that might be benefit from early chemotherapy (though in the current study the TAC is not a matter in PEC).48 However, restricted by the current topic, these questions are to be discussed in our further study. Nevertheless, as this study is the first, to our knowledge, to illustrate the optimal timing as well as duration of chemotherapy in both perioperative and postoperative settings, some of this information may only be of limited importance.
In conclusion, our analysis indicated that six or more cycles of PEC or POC alone produced a marginal and significant benefit on OS, respectively. An increasing trend in the HR for survival risk was found for TAC in patients with POC, while a decreasing trend was found in the number of cycles among the whole sample of patients. Patients who received six cycles of PEC or POC tended to have maximum survival outcomes. A prospective design is warranted to confirm our findings.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, Z.Y.L, upon reasonable request.
Ethics Approval
The Ethics Committee of Peking University Cancer Hospital approved this study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consents were obtained from all patients for being included in the study. This study does not involve animal study.
Funding
This work was funded by Beijing Municipal Health Commission (DFL20181103 and ZYLX201701), and Clinical Medicine Plus X - Young Scholars Project, Peking University.
Disclosure
All authors declare that they have no conflicts of interest.
References
1. Feng R-M, Zong Y-N, Cao S-M, Xu R-H. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun. 2019;39(1):22. doi:10.1186/s40880-019-0368-6
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Eng J Med. 2007;357(18):1810–1820. doi:10.1056/NEJMoa072252
4. Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. doi:10.1056/NEJMoa010187
5. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi:10.1056/NEJMoa055531
6. Bang Y-J, Kim Y-W, Yang H-K, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a Phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321. doi:10.1016/S0140-6736(11)61873-4
7. Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, Phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. doi:10.1016/S0140-6736(18)32557-1
8. Badgwell B, Das P, Ajani J. Treatment of localized gastric and gastroesophageal adenocarcinoma: the role of accurate staging and preoperative therapy. J Hematol Oncol. 2017;10(1):149. doi:10.1186/s13045-017-0517-9
9. Xiao H, Zhou H, Zhang P, et al. Association among the prognostic nutritional index, completion of adjuvant chemotherapy, and cancer-specific survival after curative resection of stage II/III gastric cancer. Eur J Clin Nutr. 2020;74(4):555–564. doi:10.1038/s41430-019-0502-1
10. Park HS, Jung M, Kim HS, et al. Proper timing of adjuvant chemotherapy affects survival in patients with stage 2 and 3 gastric cancer. Ann Surg Oncol. 2015;22(1):224–231. doi:10.1245/s10434-014-3949-2
11. Qu J-L, Qu X-J, Li X, et al. Early initiation of fluorouracil-based adjuvant chemotherapy improves survival in patients with resectable gastric cancer. J BUON. 2015;20(3):800–807.
12. Huang SM, Chen YC, Chen WY, et al. Optimal timing for postsurgical adjuvant therapy in patients with gastric cancer: a propensity score matching study. J Cancer. 2019;10(2):332–340. doi:10.7150/jca.27753
13. Brenkman HJF, van Putten M, Visser E, et al. Timing of postoperative chemotherapy in patients undergoing perioperative chemotherapy and gastrectomy for gastric cancer. Surg Oncol. 2018;27(3):421–427. doi:10.1016/j.suronc.2018.05.026
14. Qu J-L, Li X, Qu X-J, et al. Optimal duration of fluorouracil-based adjuvant chemotherapy for patients with resectable gastric cancer. PLoS One. 2013;8(12):e83196. doi:10.1371/journal.pone.0083196
15. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15(1):9–20. doi:10.6004/jnccn.2017.0003
16. Li Z, Wang Y, Shan F, et al. ypTNM staging after neoadjuvant chemotherapy in the Chinese gastric cancer population: an evaluation on the prognostic value of the AJCC eighth edition cancer staging system. Gastric Cancer. 2018;21(6):977–987. doi:10.1007/s10120-018-0830-1
17. UDo H, Services H. Common terminology criteria for adverse events (CTCAE) version 4.0. Natl Cancer Inst. 2009;4(03).
18. Harrell JFE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer; 2015.
19. Noh SH, Park SR, Yang H-K, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(12):1389–1396. doi:10.1016/S1470-2045(14)70473-5
20. Cirera L, Balil A, Batiste-Alentorn E, et al. Randomized clinical trial of adjuvant mitomycin plus tegafur in patients with resected stageIII gastric cancer. J Clin Oncol. 1999;17(12):3810–3815. doi:10.1200/JCO.1999.17.12.3810
21. Lim L, Michael M, Mann GB, Leong T. Adjuvant Therapy in Gastric Cancer. J Clin Oncol. 2005;23(25):6220–6232.
22. Kilic L, Ordu C, Yildiz I, et al. Current adjuvant treatment modalities for gastric cancer: from history to the future. World J Gastrointest Oncol. 2016;8(5):439–449. doi:10.4251/wjgo.v8.i5.439
23. Cai Z, Yin Y, Yin Y, et al. Comparative effectiveness of adjuvant treatments for resected gastric cancer: a network meta-analysis. Gastric Cancer. 2018;21(6):1031–1040. doi:10.1007/s10120-018-0831-0
24. Sun J, Ren Z, Sun X, Hou H, Li K, Ge Q. Efficacy and safety comparison of chemotherapies for advanced gastric cancer: a network meta-analysis. Oncotarget. 2017;8(24):39673–39682. doi:10.18632/oncotarget.17784
25. Wang F-H, Shen L, Li J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39(1):10. doi:10.1186/s40880-019-0349-9
26. Lee K-W, Zang DY, Ryu M-H, et al. Comparison of efficacy and tolerance between combination therapy and monotherapy as first-line chemotherapy in elderly patients with advanced gastric cancer: study protocol for a randomized controlled trial. Contemp Clin Trials Commun. 2017;8:55–61. doi:10.1016/j.conctc.2017.08.006
27. Zhao J-H, Gao P, Song Y-X, et al. Which is better for gastric cancer patients, perioperative or adjuvant chemotherapy: a meta-analysis. BMC Cancer. 2016;16(1):631. doi:10.1186/s12885-016-2667-5
28. Lee C-K, Jung M, Kim HS, et al. S-1 based doublet as an adjuvant chemotherapy for curatively resected stage iii gastric cancer: results from the randomized Phase III POST trial. Cancer Res Treat. 2019;51(1).
29. Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140(2):319–328. doi:10.1007/s00432-013-1563-5
30. Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–221. doi:10.1016/S1470-2045(08)70035-4
31. Cates JMM. Modeling continuous prognostic factors in survival analysis: implications for tumor staging and assessing chemotherapy effect in osteosarcoma. Am J Surg Pathol. 2018;42(4):485–491. doi:10.1097/PAS.0000000000000995
32. Petrelli F, Zaniboni A, Ghidini A, et al. Timing of adjuvant chemotherapy and survival in colorectal, gastric, and pancreatic cancer. A systematic review and meta-analysis. Cancers. 2019;11(4):550. doi:10.3390/cancers11040550
33. Lu H, Zhao B, Zhang J, et al. Does delayed initiation of adjuvant chemotherapy following the curative resection affect the survival outcome of gastric cancer patients: a systematic review and meta-analysis. Eur J Surg Oncol. 2020;46(6):1103–1110. doi:10.1016/j.ejso.2020.01.013
34. Wang X-Z, Zeng Z-Y, Ye X, Sun J, Zhang Z-M, Kang W-M. Interpretation of the development of neoadjuvant therapy for gastric cancer based on the vicissitudes of the NCCN guidelines. World J Gastrointest Oncol. 2020;12(1):37–53. doi:10.4251/wjgo.v12.i1.37
35. Brenkman HJF, Ruurda JP, Verhoeven RHA, van Hillegersberg R. Safety and feasibility of minimally invasive gastrectomy during the early introduction in the Netherlands: short-term oncological outcomes comparable to open gastrectomy. Gastric Cancer. 2017;20(5):853–860. doi:10.1007/s10120-017-0695-8
36. Ychou M, Boige V, Pignon J-P, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–1721. doi:10.1200/JCO.2010.33.0597
37. Zhang W-Y, Zhang W-J, Bai Y, et al. Impact of adjuvant chemotherapy cycles on prognosis of resectable stomach cancer: a retrospective analysis. Asian Pac J Cancer Prev. 2013;14(1):381–386. doi:10.7314/APJCP.2013.14.1.381
38. Alderson D, Cunningham D, Nankivell M, et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol. 2017;18(9):1249–1260. doi:10.1016/S1470-2045(17)30447-3
39. Petrillo A, Pompella L, Tirino G, et al. Perioperative treatment in resectable gastric cancer: current perspectives and future directions. Cancers. 2019;11(3):399. doi:10.3390/cancers11030399
40. Hebbar M, Chibaudel B, André T, et al. FOLFOX4 versus sequential dose-dense FOLFOX7 followed by FOLFIRI in patients with resectable metastatic colorectal cancer (MIROX): a pragmatic approach to chemotherapy timing with perioperative or postoperative chemotherapy from an open-label, randomized phase III trial. Ann Oncol. 2015;26(2):340–347.
41. Prendergast EN, Holzapfel M, Mueller JJ, et al. Three versus six cycles of adjuvant platinum-based chemotherapy in early stage clear cell ovarian carcinoma - A multi-institutional cohort. Gynecol Oncol. 2017;144(2):274–278. doi:10.1016/j.ygyno.2016.12.004
42. Tantoy IY, Cooper BA, Dhruva A, et al. Quality of life of patients with gastrointestinal cancers undergoing chemotherapy. Qual Life Res. 2018;27(7):1865–1876. doi:10.1007/s11136-018-1860-1
43. Lam SW, Wai M, Lau JE, McNamara M, Earl M, Udeh B. Cost-effectiveness analysis of second-line chemotherapy agents for advanced gastric cancer. Pharmacotherapy. 2017;37(1):94–103. doi:10.1002/phar.1870
44. Chikhladze S, Lederer A-K, Kousoulas L, et al. Adjuvant chemotherapy after surgery for pancreatic ductal adenocarcinoma: retrospective real-life data. World J Surg Oncol. 2019;17(1):185. doi:10.1186/s12957-019-1732-3
45. Coimbra FJF, de Jesus VHF, Ribeiro HSC, et al. Impact of ypT, ypN, and adjuvant therapy on survival in gastric cancer patients treated with perioperative chemotherapy and radical surgery. Ann Surg Oncol. 2019;26(11):3618–3626. doi:10.1245/s10434-019-07454-0
46. Jiang D, Wang H, Song Q, et al. Comparison of the prognostic difference between ypTNM and equivalent pTNM stages in esophageal squamous cell carcinoma based on the 8th edition of AJCC classification. J Cancer. 2020;11(7):1808–1815. doi:10.7150/jca.34567
47. Ji J, Shen L, Li Z, et al. Perioperative chemotherapy of oxaliplatin combined with S-1 (SOX) versus postoperative chemotherapy of SOX or oxaliplatin with capecitabine (XELOX) in locally advanced gastric adenocarcinoma with D2 gastrectomy: a randomized phase III trial (RESOLVE trial). Ann Oncol. 2019;30:v877.
48. Li Z, Shan F, Ying X, et al. Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a randomized clinical trial. JAMA Surg. 2019;154(12):1093–1101. doi:10.1001/jamasurg.2019.3473
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


