Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
308-nm Excimer Laser Plus Platelet-Rich Plasma for Treatment of Stable Vitiligo: A Prospective, Randomized Case–Control Study
Received 29 April 2020
Accepted for publication 23 June 2020
Published 23 July 2020 Volume 2020:13 Pages 461—467
DOI https://doi.org/10.2147/CCID.S260434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yanyan Deng,1,2 Jia Li,3 Gaoyun Yang1
1Department of Dermatology, Beijing Friendship Hospital Affiliated to Capital Medical University, Beijing 100050, People’s Republic of China; 2Department of Dermatology, The Second People’s Hospital of Changzhi, Changzhi 046000, People’s Republic of China; 3Department of Dermatology, Taiyuan Central Hospital of Shanxi Medical University, Taiyuan 030009, People’s Republic of China
Correspondence: Gaoyun Yang
Department of Dermatology, Beijing Friendship Hospital Affiliated to Capital Medical University, No. 95 Yong’an Road, West City District, Beijing 100050, People’s Republic of China
Tel +86-13520713158
Email [email protected]
Purpose: 308-nm excimer laser has a confirmed treatment effect on vitiligo. Platelet-rich plasma (PRP) is an autologous preparation which contains a variety of growth factors. The effect of 308-nm excimer laser combined with PRP on vitiligo has been rarely reported. This study investigated the effect of PRP combined with 308-nm excimer laser on stable vitiligo.
Patients and Methods: A total of 60 patients with localized stable vitiligo who received treatment at Beijing Friendship Hospital and Xi’an Vitiligo Specialist Hospital between May 2019 and January 2020 were consecutively enrolled. They were equally randomized into three groups according to different treatment methods: intradermal PRP injection (group I), 308-nm excimer laser alone (group II), and 308-nm excimer laser plus PRP injection (group III). All treatments lasted for 3 months. At 3 months after treatment, clinical assessments were performed in terms of the visual analogue scale (VAS) score, repigmentation response and side effects.
Results: The VAS scores showed significant differences among the three groups (P< 0.001), with the highest score in group III, followed by group II and then group I. Repigmentation responses also showed significant differences among the groups (P< 0.001), and the best effect was observed in group III. No side effects were reported in any of the groups.
Conclusion: The effect of PRP combined with 308-nm excimer laser on stable vitiligo is significantly better than that of PRP and 308-nm excimer laser alone. It is safe and satisfactorily tolerant.
Keywords: vitiligo, laser, combined therapy, visual analogue scale, repigmentation
Introduction
Vitiligo is a chronic autoimmune disease that is characterized by depigmented skin patches due to melanocyte loss.1 It is the most common depigmenting skin disorder which affects approximately 0.5–1% of the population across the world.2 Vitiligo has a serious negative influence on patients’ self-esteem and confidence; it often occurs at exposed sites such as the face,1 which seriously affects patients’ quality of life.
Melanin metabolic abnormalities play a crucial role in the development of vitiligo.2,3 Abnormal melanin loss leads to vitiligo associated pathological processes.4 Therefore, therapies that promote melanogenesis and melanocyte proliferation have been considered as the most promising treatment methods for vitiligo.5
308-nm excimer laser is monochromatic light at a specific wavelength and is formed by dimerides of xenon (8 electrons at the outmost layer) and chlorine (7 electrons at the outmost layer) after activation and continuous pulsatile release under the action of electric current.6 In 2001, Baltás and Nagy utilized 308-nm excimer laser in the treatment of vitiligo for the first time, and definite treatment effect was achieved.7 Later on, the application of 308-nm excimer laser alone in vitiligo treatment has been reported.8 Spencer et al used 308-nm excimer laser alone to treat 29 skin lesions of 18 vitiligo patients; after 6 times of treatment, 57% of 23 skin lesions (12 patients) exhibited different degrees of repigmentation; after 12 times of treatment, an effective rate of 82% was obtained among 11 lesions of 6 patients.8 However, 308-nm excimer laser alone requires a rather long treatment course (several months, or even years), and long-term irradiation poses a potential risk of carcinogenesis.9 Zhang et al used 308-nm excimer laser combined with Yiqi Qubai granules (a traditional Chinese medicine preparation) for vitiligo treatment, and a significantly better repigmentation response (the total effective rate was 56.3% (repigmentation >50%)) was obtained compared with granules alone and 308-nm excimer laser alone.10 Their results indicate that compared with 308-nm excimer laser alone, combined treatment improves the treatment effect on vitiligo, shortens the treatment course, and reduces laser accumulation. Nevertheless, more combined methods need to be investigated to further improve treatment effect on vitiligo.
Platelet-rich plasma (PRP) is a concentrate that is obtained from whole blood after centrifugation; PRP contains high-concentration growth factors,11 such as platelet-derived growth factors, transforming growth factor D, vascular endothelial growth factor, EGF fibrinogen, vitreous binding protein, and so on.12 PRP is extracted from autologous peripheral blood, and therefore does not lead to immune rejection. To date, PRP has been extensively applied in orthopedics,13 plastic surgery,14 and other fields. However, its combination with 308-nm excimer laser in the treatment of vitiligo has seldom been investigated.
Based on the abovementioned context, we conducted this prospective, case–control study to investigate the safety and effectiveness of 308-nm excimer laser combined with autologous PRP in the treatment of stable vitiligo.
Patients and Methods
Patients
In this prospective study (clinical registration no., CHiCTR1900024173), 60 adult patients with stable vitiligo who received treatment at Beijing Friendship Hospital affiliated to Capital Medical University and Xi’an Vitiligo Specialist Hospital between May 2019 and January 2020 were consecutively recruited. The diagnosis of stable vitiligo was in accordance with the Consensus on the Diagnosis and Treatment of Vitiligo15 based on the vitiligo disease activity (VIDA) score,16 with confocal laser scanning microscopy17 and dermoscopy as auxiliary diagnostic tools. The age of the patients ranged from 18 years to 65 years, with an average of 40.35±11.70 years, and the male/female ratio was 1:1.3. According to Fitzpatrick skin typing,18 all lesions were type III–IV. No new lesion or enlargement of the original lesion occurred within 12 months before the treatment. Patients that met any of the following criteria were excluded from this study:19,20 1) being pregnant; 2) receiving medication or laser treatment within 6 months before this recruitment; and 3) with bleeding disorder, a history of keloid formation, isomorphic reaction, a photosensitive history, and epilepsy.
The procedures of this trial were conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Beijing Friendship Hospital affiliated to Capital Medical University (approval no., 2019-P2-074-01). Written informed consent was obtained from each participant.
Grouping
According to different treatment methods, the patients were equally randomized into Group I (intradermal PRP injection alone), Group II (308-nm excimer laser alone) and Group III (intradermal PRP injection combined with 308-nm excimer laser) using the envelope method. Specifically, a total of 60 random numbers were generated with SAS software, which were equally divided into three groups. The information about the intervention protocols for each group was sealed in a light-proof envelope. After recruitment, patients were numbered and grouped according to the numbers written on the covers of the envelopes. They were treated in accordance with the protocols contained in the corresponding envelope. Before and at 3 months after treatment, photos were taken with a Nikon D5300 camera (Nikon Corporation, Japan).
PRP Preparation
PRP is a platelet concentrate whose concentration is 4–8 times that of the whole blood.21 PRP was prepared under a strict aseptic condition according to the literature.22–24 Up to 10 mL of fresh blood was collected from the patient into a sodium citrate vacutainer. After centrifugation at 1500 rpm for 6 min, the intermediate layer of the sample (approximately 5% of the total volume) was collected. Centrifugation at 2500 rmp was then performed for 15 min. The lower layer of the solution was collected into a 1-mL insulin syringe. Calcium chloride (at approximately 1/9 of the volume of the PRP part) was added as an activator. The solution was prepared for later use.
Treatment Methods
Group I
The patients received 3 sessions of PRP injection, with an interval of 1 month. At 40 min before injection, anesthetic cream (EMLA cream) was applied to the lesion. A 30G needle was used for superficial intradermal microinjections of the PRP preparation, with 0.1 mL per injection and 1 cm spaced apart.11
Group II
Irradiation was performed with XtracVelocity 400i-308 nm excimer laster therapeutic system (PhotoMedex, San Francisco, CA, USA). The output frequency, spot size, and the maximum energy and energy density of single pulse were set at 308 nm, 2 cm × 2 cm, 10 mJ, and 3.8 mJ/cm2, respectively. Before treatment, initial minimal erythematous dose (MED) was determined for each patient according to erythematous response at the test site 24 h after the test. Based on age and the initial obtained MED, in combination with post-irradiation response, the dose was adjusted: If erythema appeared after irradiation but lasted for less than 24 h, the dose was increased by 50%; if erythema lasted for more than 24 h but less than 48 h, the dose was maintained; and if erythema lasted for more than 48 h or blisters appeared, the treatment was temporarily discontinued, and after the blisters disappeared, irradiation was resumed with a dose decreased by 50%. The patient underwent irradiations twice per week (the irradiation duration was determined according to the conditions of the skin lesion), with 24 times in total.
Group III
Considering that PRP can stabilize melanocytes and excimer laser can promote the proliferation of melanocytes,25 PRP was injected 30 min before each monthly session of laser treatment. Other procedures were the same as were performed for groups I and II.
Clinical Assessment
At 3 months after treatment, clinical assessments were performed in terms of patient’s satisfaction, repigmentation response and side effects.
Patient’s Satisfaction
Patients rated their satisfaction using a 10-point visual analogue scale (VAS), according to which, 0 point was described “not satisfied at all,” whereas 10 was described “completely satisfied”.25
Repigmentation Assessment
Repigmentation was assessed by two independent experienced dermatologist, who were not aware of the objective of this study. Repigmentation evaluation was based on the following criteria: excellent for 75–100% repigmentation, good for 50–75%, moderate for 25–50%, and mild for <25%.22,26
Side Effects
The patients were informed to report any complications, such as erythema, pain, ulceration, burning sensation, ecchymosis, infection, postinflammatory hyperpigmentation, and allergic manifestations.
Statistical Analysis
SPSS 22.0 was used for data processing. Measurement data were presented as the mean ± standard deviation (SD). Data that satisfied normal distribution and homogenous variances were analyzed using analysis of variance. Otherwise, a Kruskal–Wallis H-test was performed. Numeration data were presented as frequencies or percentages, and analyzed using the chi-square test. Ordinal categorical numeration data were analyzed using the Kruskal–Wallis H-test. For pairwise comparisons among groups, the Bonferroni method was used for corrections. A difference at p<0.05 was considered statistically significant.
Results
General Data
The general data of the three groups are summarized in Table 1. The three groups did not show a significant difference in age (F=0.374, p=0.689). They did not show a significant difference in the sex composition, either (χ2=0.950, p=0.622). No significant differences in family history and lesion types were observed among the groups (p>0.05). No significant differences in the localization of the lesions were observed among the groups (x2=1.425, p=0.994; Figure 1).
 |
Table 1 General Data of the Three Groups |
 |
Figure 1 Locations of the lesions in different groups. |
VAS Scores
The VAS scores of the three groups are summarized in Table 2. Group III showed significant differences compared with group I and group II (p<0.001 and p=0.045, respectively). Although group II had a higher VAS score than group I, no significant difference was observed (p=0.23).
 |
Table 2 VAS Scores of the Three Groups |
Repigmentation
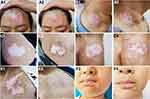
The treatment effects of the three groups are summarized in Table 3, and some typical examples of the repigmentation responses in different groups are shown in Figure 2. In group I, the numbers of the patients that presented with mild, moderate, good, and excellent repigmentation were 8, 7, 5, and 0, respectively, with a total effective rate of 25.00%. In group II, the numbers of the patients were 2, 11, 5, and 2, respectively, with a total effective rate of 35.00%. In group III, the numbers were 1, 3, 8, and 8, respectively, with a total effective rate of 80.00%. The repigmentation response in group III was significantly better than that in group I (adjusted p<0.001) and in group II (adjusted p=0.045). Group III also showed a significantly higher total effective rate than group I (adjusted p<0.001) and group II (adjusted p=0.036). Group II did not show a significant difference in the repigmentation response and total effective rate compared with group I (both p>0.05).
 |
Table 3 Treatment Effects in Different Groups |
Side Effects
No side effects were reported in any of the groups.
Discussion
To date, a variety of treatment methods for vitiligo have been proposed. However, most of them cannot achieve satisfactory treatment effect. In this study, we investigated the curative effect of intradermal PRP injection combined with 308-nm excimer laser as well as its safety in the treatment of vitiligo.
Our studies showed that the VAS score of group III after treatment was 8.05±2.24, which was significantly higher than that of group I and of group II. Furthermore, in this group, good repigmentation (>50%) was observed among 40% of the patients and excellent repigmentation (>75%) also among 40% of the patients, with a total effective rate of 80.00%. Repigmentation response of group III was significantly better than that of group I and of group II. Khattab et al conducted a study to investigate the effect of PRP injection combined with 308-nm excimer laser on vitiligo.25 At 3 months after treatment with the combined method, 50% of the patients showed repigmentation >50% and 34.6% showed repigmentation >75%.25 Our results were basically consistent with theirs. However, there were some differences. First, Khattab et al did not include a PRP lone group. Second, their treatment course was 4 months, whereas ours was 3 months. Third, in this study, the percentages of the patients showing excellent repigmentation response in the combined treatment group and 308-nm excimer laser group were higher than those in their study (40% vs 34.6% and 10% vs 0%, respectively). Presumably, these inconsistencies were due to different sample sizes between our study and theirs. Goldinger et al used 308-nm excimer laser combined with calcipotriol to treat vitiligo; however, this combination did not significantly improve the effective rate compared with 308-nm excimer laser alone.27 Tacalcitol, even at a high concentration, had a limited effect on vitiligo when combined with 308-nm excimer laser.28 Our results indicate that the combination of 308-nm excimer laser with intradermal PRP injection can obtain a satisfactory treatment effect on vitiligo.
Presumably, the mechanisms underlying the effect of the combined therapy used in this study are as follows. 308-nm excimer laser stimulates melanocytes to synthesize melanin in vivo; in the meantime, the laser rapidly induces the apoptosis of infiltrating pathological T lymphocytes in skin lesions, thereby preventing melanocytes from being destroyed.9 In the treatment of vitiligo, PRP forms and secretes cytokines into keratinocytes and melanocytes to suppress the release of inflammatory cytokines and apoptosis of melanocytes; it has anti-inflammatory effect, which suppresses the release of cytokines such as interleukin-1, interferon-c, and tumor necrosis factor-a (these cytokines have great roles in the pathogenesis of vitiligo).29–33 PRP contains high-concentration growth factors,11 which promote the proliferation of keratinocytes and fibroblasts and enhance their interactions with melanocytes.34 In the treatment of vitiligo, 308-nm excimer laser and PRP exert synergetic actions, which suppress the release of cytokines on the one hand and stabilize melanocytes on the other. In addition, apart from improving repigmentation response of vitiligo patients, intradermal PRP injection also shortens the duration of exposure to excimer laser irradiation, thereby reducing the risk of carcinogenesis. Meanwhile, PRP is autologous, and therefore, it is safe and patients’ tolerance is satisfactory. In addition, antibody formation does not need to be particularly considered in the clinical application of PRP.
This study suffered from the following limitations. The sample size was small and all the patients involved in this study were from the same center. Therefore, multicentric studies with a larger sample size remain to be conducted in the future. In addition, the treatment effect in this study was assessed 3 months after treatment. To validate the outcomes of this study, long-term follow-ups should also be carried out.
Conclusions
In conclusion, 308-nm excimer laser combined with intradermal PRP injection is an effective treatment method for stable vitiligo. The combination of PRP significantly improves the treatment effect of 308-nm excimer laser alone and greatly shortens the treatment course. 308-nm excimer laser combined with intradermal PRP injection is safe and is worth being promoted in clinical practice.
Data Sharing Statement
No additional data are available.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bergqvist C, Ezzedine K. Vitiligo: a review [published online ahead of print, 2020 Mar 10]. Dermatology. 2020;1–22. doi:10.1159/000506103
2. Abdel-Malek ZA, Jordan C, Ho T, Upadhyay PR, Fleischer A, Hamzavi I. The enigma and challenges of vitiligo pathophysiology and treatment [published online ahead of print, 2020 Mar 21]. Pigment Cell Melanoma Res. 2020. doi:10.1111/pcmr.12878
3. Li L, Liang Y, Zhang D, et al. The 308-nm excimer laser stimulates melanogenesis via the wnt/β-Catenin signaling pathway in B16 cells. J Dermatol Treat. 2019;21:1–5.
4. Wu Y, Sun Y, Qiu L, et al. A multicentre, randomized, split face and/or neck comparison of 308-nm excimer laser and 0.1% tacrolimus ointment for stable vitiligo plus intramuscular slow-releasing betamethasone for active vitiligo. Br J Dermatol. 2019;181(1):210–211. doi:10.1111/bjd.17630
5. Li L, Liang Y, Hong J, Lan L, Xiao H, Xie Z. The effectiveness of topical therapy combined with 308-nm excimer laser on vitiligo compared to excimer laser monotherapy in pediatric patients. Pediatr Dermatol. 2019;36(1):e53–e55. doi:10.1111/pde.13726
6. Wang X, Liu GY. Progress of 308 nm excimer laser combined with other methods in the treatment of vitiligo. Chin J Dermatol. 2017;50(9):695–697.
7. Baltás E, Nagy P. Repigmentation of localized vitiligo with the xenon chloride laser. Br J Dermatol. 2001;144(6):1266–1267. doi:10.1046/j.1365-2133.2001.04248.x
8. Spencer JM, Nossa R, Ajmeri J. Treatment of vitiligo with the 308 nm excimer laser: a pilot study. J Am Acad Dermatol. 2002;46:727–731. doi:10.1067/mjd.2002.121357
9. Mehraban S, Feily A. 308 nm excimer laser in dermatology. J Lasers Med Sci. 2014;5:8–12.
10. Zhang C, Zhou L, Huang J, Shi W. A combination of Yiqiqubai granule and 308-nm excimer laser in treatment of segmental vitiligo: a prospective study of 233 patients. J Dermatol Treat. 2017;28(7):668–671. doi:10.1080/09546634.2017.1303570
11. Abuaf OK, Yildiz H, Baloglu H, Bilgili ME, Simsek HA, Dogan B. Histologic evidence of new collagen formulation using platelet rich plasma in skin rejuvenation: a prospective controlled clinical study. Ann Dermatol. 2016;28(6):718–724. doi:10.5021/ad.2016.28.6.718
12. Charneux L, Demoulin C, Vanderthomment M, et al. Platelet-rich plasma (PRP) and disc lesions: a review of the literature. Neurochirurgie. 2017;63(6):473–477. (in French). doi:10.1016/j.neuchi.2017.06.002
13. Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11(1):35–44. doi:10.1038/nrrheum.2014.162
14. Badran KW, Sand JP. Platelet-rich plasma for hair loss: review of methods and results. Facial Plast Surg Clin North Am. 2018;26(04):469–485. doi:10.1016/j.fsc.2018.06.008
15. Xu AE, Gao TW. Consensus on diagnosis and treatment of vitiligo (2018). Chin J Dermatol. 2018;51(4):247–250.
16. Njoo MD, Das PK, Bos JD, Westerhof W. Association of the Kobner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1999;135(4):407–413. doi:10.1001/archderm.135.4.407
17. Xiang W, Xu A, Xu J, Bi Z, Shang Y, Ren Q. In vivo confocal laser scanning microscopy of hypopigmented macules: a preliminary comparison of confocal images in vitiligo, nevus depigmentosus and postinflammatory hypopigmentation. Lasers Med Sci. 2010;25(4):551–558. doi:10.1007/s10103-010-0764-2
18. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi:10.1001/archderm.1988.01670060015008
19. Yuan J, Chen H, Yan R, et al. Fractional CO2 lasers contribute to the treatment of stable non-segmental vitiligo. Eur J Dermatol. 2016;26(6):592–598. doi:10.1684/ejd.2016.2875
20. Abdelghani R, Ahmed NA, Darwish HM. Combined treatment with fractional carbon dioxide laser, autologous platelet-rich plasma, and narrow band ultraviolet B for vitiligo in different body sites: a prospective, randomized comparative trial. J Cosmet Dermatol. 2018;17(3):365–372. doi:10.1111/jocd.12397
21. Yuan T, Guo S, Han P, et al. Applications of leukocyte and platelet -rich plasma (L-PRP) in trauma surgery. Curr Pharm Biotechnol. 2012;13(7):1173–1184. doi:10.2174/138920112800624445
22. Kim DH, Je YJ, Kim CD, et al. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol. 2011;23:424–431. doi:10.5021/ad.2011.23.4.424
23. Ibrahim ZA, El-Ashmawy AA, Shora OA. Therapeutic effect of microneedling and autologous platelet rich plasma in the treatment of atrophic scars: a randomized study. J Cosmet Dermatol. 2017;16(3):388–399. doi:10.1111/jocd.12356
24. Kumaran MS. Platelet-rich plasma in dermatology: boon or a bane? Indian J Dermatol Venereol Leprol. 2014;80(1):5–14. doi:10.4103/0378-6323.125467
25. Khattab FM, Abdelbary E, Fawzi M. Evaluation of combined excimer laser and platelet‐rich plasma for the treatment of nonsegmental vitiligo: A prospective comparative study. J Cosmet Dermatol. 2020; 19(4): 869–877. doi:10.1111/jocd.v19.4
26. Osinubi O, Grainge MJ, Hong L, et al. The prevalence of psychological comorbidity in people with vitiligo: a systematic review and meta-analysis. Br J Dermatol. 2018;178(4):863–878. doi:10.1111/bjd.16049
27. Goldinger SM, Dummer R, Schmid P, Burg G, Seifert B, Läuchli S. Combination of 308 nm xenon chloride excimer laser and topical calcipotriol in vitiligo. J Eur Acad Dermatol Venereol. 2007;21:504–508. doi:10.1111/j.1468-3083.2006.02016.x
28. Oh SH, Kim T, Jee H, Do JE, Lee JH. Combination treatment of non-segmental vitiligo with a 308-nm xenon chloride excimer laser and topical high-concentration tacalcitol: a prospective, single-blinded, paired, comparative study. J Am Acad Dermatol. 2011;65:428–430. doi:10.1016/j.jaad.2010.12.007
29. Choi CP, Kim YI, Lee JW, Lee MH. The effect of narrowband ultraviolet B on the expression of matrix metalloproteinase-1, transforming growth factor-beta1 and type I collagen in human skin fibroblasts. Clin Exp Dermatol. 2007;32:180–185. doi:10.1111/j.1365-2230.2006.02309.x
30. Cario-Andre M, Pain C, Gauthier Y, Casoli V, Taoeb A. In vivo and in vitro evidence of dermal fibroblasts influence on human epidermal pigmentation. Pigment Cell Res. 2006;19:434–442. doi:10.1111/j.1600-0749.2006.00326.x
31. Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17:96–100. doi:10.1111/j.1600-0749.2003.00126.x
32. Dey-Rao R, Sinha AA. Interactome analysis of gene expression profile reveals potential novel key transcriptional regulators of skin pathology in vitiligo. Genes Immun. 2016;17:30–45. doi:10.1038/gene.2015.48
33. El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–669. doi:10.1902/jop.2007.060302
34. Devereaux J, Nurgali K, Kiatos D, Sakkal S, Apostolopoulos V. Effects of platelet-rich plasma and platelet-poor plasma on human dermal fibroblasts. Maturitas. 2018;117:34–44. doi:10.1016/j.maturitas.2018.09.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.