Back to Journals » International Journal of Women's Health » Volume 13
Current Perspectives of Prenatal Sonographic Diagnosis and Clinical Management Challenges of Complex Umbilical Cord Entanglement
Authors Sherer DM , Roach C, Soyemi S, Dalloul M
Received 4 November 2020
Accepted for publication 23 January 2021
Published 24 February 2021 Volume 2021:13 Pages 247—256
DOI https://doi.org/10.2147/IJWH.S285860
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
David M Sherer, Crystal Roach, Sarin Soyemi, Mudar Dalloul
The Division of Maternal-Fetal Medicine, The Department of Obstetrics and Gynecology, State University of New York (SUNY), Downstate Health Sciences University, Brooklyn, NY, USA
Correspondence: David M Sherer
Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, State University of New York (SUNY), Downstate Health Sciences University, 450 Clarkson Avenue, Box 24, Brooklyn, NY, 11203, USA
Tel +1 718 270-2081
Fax +1 718 270-4122
Email [email protected]
Abstract: Diagnosis of potential umbilical cord compromise, namely, true knots of the umbilical cord and nuchal cords has been enabled with increasing accuracy with current enhanced prenatal sonography. Often an incidental finding at delivery, the incidence of true knots of the umbilical cord has been estimated at between 0.04% and 3% of deliveries. This condition has been reported to account for a 4 to 10-fold increase of stillbirth and perinatal morbidity of 11% of cases. Nuchal cords, commonly observed at the delivery of uncompromised, non-hypoxic non-acidotic newborns occur more frequently with single nuchal cords noted in between 20% and 35% of all deliveries at term. Multiple nuchal cords are considerably less frequent, with decreasing frequencies inverse to the number of nuchal cord loops. While clearly single (and likely double) nuchal cords are almost uniformly associated with favorable neonatal outcomes, emerging data suggest that cases of ≥ 3 loops of nuchal cords are more likely to be associated with an increased risk of adverse perinatal outcome (either stillbirth or compromised neonatal condition at delivery). We define cases of a true knot of the umbilical cord, cases of ≥ 3 loops of nuchal cords, any combination of a true knot and nuchal cord, or any umbilical cord entanglement (nuchal or true knot) in the presence of a single umbilical artery, in singleton gestations as complex umbilical cord entanglement. Two concurrent developments, the increase in accuracy of prenatal sonographic diagnosis of complex umbilical cord entanglement and recent data confirming fatal compromise of the umbilical circulation in approximately 20% of cases of stillbirth, suggest that establishing governing body guidelines for reporting of potential umbilical cord compromise, and recommendation of consideration for early-term delivery of select cases, may be warranted. This commentary will address current perspectives of prenatal diagnosis and clinical management challenges of complex umbilical cord entanglement.
Keywords: prenatal ultrasound, complex umbilical cord entanglement, true knot of the umbilical cord, nuchal cord, clinical management
Introduction
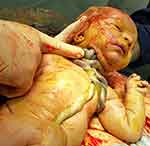
For purposes of this commentary and suggested clinical management, we propose the definition of complex umbilical cord entanglement as those cases involving prenatal sonographic diagnosis of a true knot of the umbilical cord, cases of ≥3 loops of nuchal cords, any combination of a true knot and nuchal cord, or any umbilical cord entanglement (nuchal or true knot) in the presence of a single umbilical artery, in singleton gestations. The complexity and potential occlusive hazards of this condition are depicted clearly in Figure 1.
This commentary will outline emerging data pertaining to current (and potential future) imaging diagnostic capabilities, review existing literature regarding increased adverse perinatal outcome associated with, and forward our proposed clinical management following prenatal sonographic diagnosis of complex umbilical cord entanglement.
Background
Although umbilical cord accidents preceding labor traditionally have been considered uncommon, recently published data support that as many as 20% of stillbirths at autopsy result from compromise of the umbilical cord circulation.1–3 Among 121 reported stillbirths, umbilical cord pathology was the reported main cause of third-trimester stillbirth (33.33%) and overall total stillbirth (23.97%). Similarly, umbilical cord pathology was the third most common cause of second-trimester stillbirth (16.42%).1 A Finnish study in 2017 found that “severe constricting loops or knots” were the cause of 16/98 (16.3%) of stillbirths.2 Most recently (2020), the NICHD Stillbirth Collaborative Research Network Group reported that of 496 stillbirths with the detailed cause of death analysis, using the INCODE (Initial Causes of Fetal Death) classification system 94 (19%, 95% CI 16–23%) resulted from umbilical cord abnormality, 27 (29%) either nuchal, body or shoulder umbilical cord entanglement, 26 (27%) either knots, torsions, or stricture, and 5 (5%) had a prolapse of the umbilical cord.3
In 2008, suggested histopathology criteria reflecting restriction of umbilical cord blood flow in cases of unexplained stillbirth, were forwarded by Parast et al.4 These authors correlated clinical and autopsy findings with gross and histology placental data from a series of presumptive umbilical cord accidents, proposing minimal histology findings suggestive of compromise of umbilical cord flow, which included: vascular ectasia and thrombosis within the umbilical cord, chorionic plate, and/or stem villi. Overall, of 27/62 cases where death was considered other than cord accident, only 3/62 (11%) cases met all histology criteria for umbilical cord accident (89% specificity). Of the 25 cases of stillbirth with an unknown cause of death, 13/25 (52%) met criteria for an umbilical cord accident (P = 0.0038).4
Later in a separate study in 2012, the sensitivity and specificity of these criteria were assessed in an additional review of an independent set of stillbirths.5 Histopathology findings from 26 cases (in which cord accident was considered the cause of death) and 62 cases (in which the cause of death was not an umbilical cord accident), were assessed. Of the 62 stillbirth cases in which the umbilical cord was not the cause of stillbirth, only 4 (6%) met the complete criteria for umbilical cord accident (4% specificity). Of the 26 cases with a cause of death attributed to an umbilical cord accident, 16 met the minimal criteria (62% sensitivity) and 12 met all the criteria (46% sensitivity).5 These authors thus confirmed that the above-detailed histology criteria correctly identify cases of cord accident as a cause of stillbirth with high specificity and concluded that these criteria are of potential clinical utility in establishing the diagnosis of an umbilical cord accident among stillbirths.5
The umbilical cord is a clear extension of the fetal cardiovascular system. Umbilical vessels (both umbilical arteries and umbilical vein) are protected by inherent anatomical characteristics of the umbilical cord. These characteristics include: umbilical cord length, Wharton’s jelly, two arteries, coiling, and overall suspension of the umbilical cord (critical for fetal development) in protective amniotic fluid. Cumulatively these features contribute to protective buffering of the umbilical cord from compression, shearing and twisting forces during gestation and particularly, during uterine contractions and subsequent fetal descent in the maternal pelvis and birth canal throughout labor and delivery.6 The presence of the Hyrtl anastomosis, a vascular shunt 1.5–2 cm in length located between the umbilical arteries and positioned 3 cm from the placental cord insertion (present in approximately 96% of umbilical cords), constitutes yet another protective mechanism from potential compression effects upon the paired umbilical arteries.6 The Hyrtl anastomosis equalizes pressures between the respective umbilical arteries prior to entering the placenta. The main function of this vascular anastomosis is to serve as a safety valve in the event of placental compression or potential blockage of one of the umbilical arteries. The presence of the Hyrtl anastomosis has been confirmed in a number of prenatal ultrasound studies.7–9 The “safety valve” protection from potential umbilical artery compression is clearly absent in the presence of a single umbilical artery (the most common true congenital anomaly of humans).6 It is precisely this loss of protection from potential umbilical artery compromise due to compression in fetuses with a single umbilical artery, which we address in extending the definition of complex umbilical cord entanglement to include any umbilical cord entanglement (nuchal or true knot) in the presence of a single umbilical artery.
Unimpaired blood flow through the umbilical cord may be in jeopardy of potential compromise in the presence of true knots of the umbilical cord or nuchal cord(s) or rarely a combination of both. A multitude of factors predispose to the formation of nuchal cord(s) and true knot(s) of the umbilical cord and include: long umbilical cords, polyhydramnios, gestational diabetes, multiparity, post-term pregnancy, excessive fetal movements, marginal umbilical cord insertion and interestingly, male fetuses.9–12
Although true knots of the umbilical cord are relatively uncommon (fewer than 1% of singleton deliveries), these are associated with a 4- to 10-fold increased incidence of stillbirth. True knots of the umbilical cord often remain undetected at prenatal sonography due to the inability of ultrasound to depict the entire length of the umbilical cord. In contrast, nuchal cord(s) occur more frequently than true knots of the umbilical cord and are considerably more amenable to precise prenatal diagnosis. The incidence of nuchal cords increases with increasing gestational age, occurring in between 15.8% and 30% of all singleton deliveries.13–15 In 2005 Schäffer et al found an incidence of nuchal cords in term and post-term deliveries of 33.7% and 35%, respectively.16 It appears that the increased incidence of nuchal cord towards term may reflect increasing fetal activity concurrent with decreasing amniotic fluid volume17,18 Multiple nuchal cords occur less frequently (5% or lower for double nuchal cords), and overall, the incidence is inverse to the increasing number of nuchal cord loops.16,19,20, Dipple reported the incidence of a quadruple nuchal cord of 0.1% of all deliveries.20 Unusual cases involving “high order” multiple loops of nuchal cord of 5,6,7,8,9 and 10 nuchal loops have all been documented.12,21–26 Of these anecdotal cases, the cases with 5, and 10 loops of nuchal cord, respectively, were associated with stillbirth most likely due to the multiple nuchal cords.12,26 In the cases with 6, 7, 8, and 9 reported loops of nuchal cords, respectively, cesarean delivery was necessitated, resulting live born neonates.21–25
Imaging
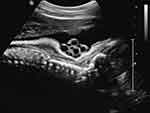
As mentioned earlier, nuchal cord(s) are depicted with relative ease at prenatal sonography. The fixed anatomical location of nuchal cords (fetal neck) is uniformly assessed directly with ultrasound throughout gestation, and is unaffected by potential confounding factors including: maternal obesity or amniotic fluid volume abnormalities (either oligohydramnios or polyhydramnios). Initially, prenatal sonographic diagnosis of nuchal cord was reported with real-time ultrasonography. Ranzini et al, in 1999 described the classic details of the “divot sign”, in which a marked “scalloping” indentation of subcutaneous tissue in the posterior aspect of the fetal neck, resulting from pressure of the nuchal cord upon the fetal neck, was observed (Figure 2).27
Both improved sonographic resolution and widespread application of color and power Doppler, and three-dimension (3D) technologies, have resulted in increasing accuracy of prenatal sonographic diagnosis of nuchal cord(s), (Figures 2–4)(Figures 3–8).28–31 Current reported sensitivity and specificity rates of prenatal diagnosis of nuchal cords range between 80% and between 96.8% and 87% and 96%, respectively.28–31 Nuchal cord(s) represent a dynamic condition in-utero and may form (single or multiple loops) and alternatively undergo spontaneous resolution. This dynamic status (formation and spontaneous reduction of nuchal cords) likely explains why 100% prenatal sensitivity and specificity values in the prenatal sonographic diagnosis of this condition at delivery are unlikely.
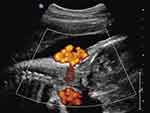 |
Figure 3 Sagittal imaging of the fetal neck. Power Doppler imaging depicting a quadruple nuchal cord depicted in Figure 2. Note that each of the larger umbilical veins is accompanied by two (smaller caliber) umbilical arteries, respectively. |
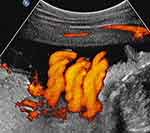 |
Figure 4 Sagittal image of the fetal neck. Fetal cranium is to the right of the image. Power Doppler depicting a triple nuchal cord. |
In 2004, Ramon y Cajal and Martinez with color Doppler imaging reported a new sonographic sign of true knot of the umbilical cord in five cases, in which an incomplete circle of umbilical cord surrounded the centric, axial or transverse section through the umbilical cord, “en face” in which the umbilical vessels were clearly depicted within the incomplete loop of cord.32
Prenatal sonographic diagnosis of a true knot of the umbilical cord is reported with increasing accuracy with wide clinical utilization of color and power and three-dimension sonographic technologies, with reported sensitivities of between 62% (5/8)33 and 100% (3/3 and 5/5, respectively) in limited case series32,35 Prenatal sonographic diagnosis of true knots of the umbilical cord is considerably more challenging than that of nuchal cord(s). This reflects a number of factors including; the inability to depict the umbilical cord throughout its entire length and the fact that a true knot may be obscured by the fetus and thus inaccessible to the sonographic beam. In addition, potential “clumping or clustering” of loops of umbilical cord may lead to a possible false-positive diagnosis. The accuracy of sonographic prenatal diagnosis of true knot the umbilical cord clearly depends on the presence of the knot in a visible area of sonographic insonation, awareness and experience of the sonographer, and the policy of the given sonography unit in reporting this diagnosis.
Complex Umbilical Cord Entanglement
As mentioned earlier, increased adverse outcomes associated with true knot(s) of the umbilical cord are well established. In contrast, abundant evidence supports the usual lack of adverse neonatal outcome associated with single or double loops of nuchal cord.15,35,36 Recent reports, however, suggest a considerable increase in adverse neonatal outcome with ≥3 loops of nuchal cord.37–41
In 1995, Jauniaux et al compared perinatal outcomes of 550 fetuses with nuchal cord at delivery with control fetuses matched for gestational age, maternal age and parity. These authors divided the fetuses with nuchal cords into two separate groups, those with single and those with multiple nuchal cords, respectively.37 Both groups exhibited similar mortality rates, umbilical artery pH levels <7.16, umbilical venous pH < 7.2 and Apgar scores of below 7 at 5 and 10 minutes.37 These authors however did report a significantly higher incidence of 1-minute Apgar scores below 7, meconium, emergency Cesarean delivery, neonatal resuscitation and NICU admission among the nuchal cord group. Of note, multiple nuchal cords were the primary factor for the higher incidence of these complications and notably, the etiology for the three cases of stillbirth, whom all presented in the preceding week with decreased fetal movements.37
A number of publications concur with less than optimal outcomes regarding multiple (≥3) nuchal cords. Recently, in 2019 Schreiber et al presented a retrospective cohort analysis of 42,798 women with singleton, vertex vaginal births between 24 and 43 weeks’ gestation, reporting 3809 (8.9%) women with single nuchal cords, 1035 (2.42%) with double nuchal cord and 258 (0.6%) with three loops.38 These authors reported that nuchal cord with three loops compared to no nuchal cord was associated with a higher incidence of stillbirth (1.9%), Apgar scores lower than 7 at 1 and 5 minutes (7.4%, and 2.3%, respectively), and a 17.5% higher rate of operative vaginal births. Fetuses with a nuchal cord of 2 or 3 loops were associated with considerably higher incidences of impaired fetal growth (10.2% and 11.6%, respectively). Multiple logistic regression analysis revealed that nuchal cord with three loops was an independent risk factor for operative vaginal delivery and 1-minute Apgar scores lower than 7. Of note, single nuchal cords were not associated with adverse perinatal outcomes.38
In 2018, Mariya et al analyzed 2156 term deliveries, assessing in addition to the nuchal cord, neonates with trunk and limb cord entanglement, classifying cases into three groups, no loop (n=1458), single loop (n=594), and multiple loops (n=104).39 Umbilical artery pH levels and base excess levels were considered significantly “unfavorable” (P=0.002, and P<0.001, respectively) among entanglement cases notably in the multiple loops group.39 The need for oxygen supplementation at delivery was significantly higher among neonates in the multiple loops group (P<0.001).30
In 2008, Önderglu et al determined that oligohydramnios, impaired fetal growth, intrapartum abnormalities and 1-minute Apgar scores lower than 7 were not significantly different between cases with versus cases without nuchal cords.40 However, these authors noted that umbilical cord pH (7.32 versus7.30, P=0.048), PO2 (37.4 ± 18.1, versus 31.7 ± 14.4, P=0.01) and O2 saturation (57 ± 2.8 versus 48.3 ± 20.4 P=0.005) were significantly lower in the nuchal cord group. In addition, the percentage of 1-minute Apgar scores lower than 7 were significantly higher in the group of neonates with multiple nuchal cords (31.3% versus 15.6%, P= 0.04). These authors suggested that while the presence of a single nuchal cord may negatively affect umbilical cord gases albeit without significant perinatal complications, the presence of multiple nuchal cords may considerably increase the likelihood of intrapartum complications and lower Apgar scores.40
In 2012, Hoh et al determined that multiple nuchal cords (≥3 loops) were associated with a higher incidence of intrapartum non-reassuring fetal heart rate, meconium-stained amniotic fluid, neonatal intensive care unit admission, and intrapartum emergency Cesarean delivery.41
In 2020, Sepulveda reported 10 singleton fetuses with triple nuchal cord after 24 weeks’ gestation (with a calculated prevalence of 1 in 506 or 0.2% of deliveries).42 Four cases detected after 36 weeks’ gestation underwent Cesarean delivery and a triple nuchal cord was confirmed at delivery. Women with prenatal sonographic diagnosis of triple nuchal cord at below 36 weeks’ gestation were followed expectantly. In 83% of these cases, the nuchal cord spontaneously reduced by at least one loop. Of 8/10 cases delivered by Cesarean only in two of these deliveries, the presence of the triple nuchal cord was the sole indication for Cesarean.42
Two recent reports have addressed prenatal sonographic diagnosis of coexisting true knot of the umbilical cord and nuchal cord.34,43 In 2017, we reported three third-trimester cases in which the true knot of the umbilical cord was located within the loop of the nuchal cord itself (Figure 5).34 In all three cases following administration of intramuscular antenatal steroids and admission for continuous fetal monitoring, prolonged fetal bradycardia led to emergency Cesarean delivery of non-hypoxic, non-acidotic, uncompromised infants. At delivery, all three of these uncompromised neonates exhibited coexisting nuchal cords with a true knot of the umbilical cord located precisely as depicted by prenatal ultrasound, within the nuchal cord loop.34
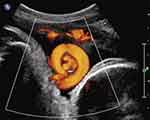 |
Figure 5 Sagittal imaging of the fetal neck. Fetal cranium is to the right of the image. Power Doppler imaging depicts coexisting true knot of the umbilical cord located within a nuchal cord (note the umbilical vein and two arteries seen “en face” within the almost complete umbilical cord circle). Reproduced from Sherer DM, Dalloul M, Ward K, et al. Coexisting true umbilical cord knot and nuchal cord: possible cumulative increased risk of adverseperinatal outcome. Ultrasound Obstet Gynecol. 2017;50(3):404–405. Copyright © 2016 ISUOG. Published by John Wiley & Sons Ltd.34 |
Further caution is likely warranted in the event of complex umbilical cord entanglement in the presence of a single umbilical artery, in that these fetuses lack the inherent decompression potential of the second (paired) umbilical artery. We previously observed the potential effect of lack of such an inbuilt compensatory system of a single umbilical artery in a fetus with a double nuchal cord who required emergency Cesarean delivery at 31 weeks’ gestation due to prolonged fetal bradycardia (following administration for fetal testing and intramuscular steroids to decrease overall prematurity-associated neonatal morbidities).44 We postulated that intermittent compression or occlusion of a single umbilical artery might impair return of unoxygenated fetal blood to the placenta, beyond that which may occur following compression of one of the two umbilical arteries in a normally structured umbilical cord (with an intact second umbilical artery for maintaining delivery of deoxygenated fetal arterial blood to the placenta).44 As mentioned earlier in this commentary, it is precisely this loss of protection from potential umbilical artery compromise due to compression in fetuses with a single umbilical artery, which we address in extending the definition of complex umbilical cord entanglement to include any umbilical cord entanglement (nuchal or true knot) in the presence of a single umbilical artery.
It is important to consider the (rarely) reported possible association between complex umbilical cord entanglement and fetal growth restriction.45–47 This is of special importance when the diagnosis of complex umbilical cord entanglement occurs in prematurity (or extreme prematurity) in that diagnosis of the latter may precede the fetal growth restriction, which may only become established/recognized considerably later in gestation. Delivery in prematurity may in these rare occurrences, become indicated due to matters relating to fetal growth restriction rather than the associated complex umbilical cord entanglement (or a combination of both of these conditions).45–47
Clinical Management
Although the precise percentage remains undetermined, it is clear that many (if not most) cases of fetuses with complex umbilical cord entanglement will not be affected and will not require delivery by Cesarean. Notwithstanding, it is clearly evident that the likelihood of potential stillbirth or neonatal compromise is increased among cases with complex umbilical cord entanglement. The question, therefore, remains, what actions, if any, are to be taken upon prenatal sonographic diagnosis of these conditions? Do we inform the patient? Is increased fetal surveillance warranted? Should delivery be considered? And if so, at what gestational age?48–50
In our unit in an inner-city teaching hospital, we are simply unwilling to withhold the potentially critical prenatal diagnosis of complex umbilical cord entanglement from our patients, as others have proposed/inferred.51–53 We therefore uniformly inform our patients of this condition in real-time upon diagnosis, irrespective of gestational age. Our patients are counseled in a fashion tapered to the details of their unique complex umbilical cord entanglement. Diagnosis in prematurity also requires detailed follow up sonographic assessments of fetal growth due to the previously discussed uncommon association of complex umbilical cord entanglement and fetal growth restriction.45–47
Stillbirth in cases of umbilical cord accidents is attributed to obstruction of umbilical venous return from the placenta.6 It is therefore important to consider that in contrast to many other etiologies of stillbirth umbilical cord accidents are likely acute, and thus affected fetuses are less likely to benefit from the compensatory system of redistribution of oxygenated blood towards essential fetal organs (central nervous system, heart and adrenal glands), as may occur in association with uteroplacental insufficiency.
It is our experience that patients far prefer to be informed and actively participate in decision-making regarding the potentially ominous prenatal sonographic finding of complex umbilical cord entanglement. Recognizing the regretful potential inadequacies/deficiencies of fetal testing (inability to completely avoid potential stillbirth especially in cases of umbilical cord compromise) given the choice, our patients almost uniformly prefer to initiate delivery ≥37 weeks’ gestation in the absence of fetal compromise, unless warranted earlier (with other than pristine fetal testing of fetal growth restriction). Not disregarding the anxiety associated with the diagnosis of complex entanglement of the umbilical cord our patients find themselves empowered and appreciative of the option to be assured of the early term delivery of a live-born infant without the (possibly misleading/false) reassurance provided by intermittent (twice weekly) fetal testing until 39 weeks’ gestation. At gestational ages <37 weeks’ we recommend daily fetal movement assessment, and twice-weekly fetal testing, while maintaining a low threshold for admission in select cases (subjective decreased fetal movements, growth restriction, oligohydramnios) for IM antenatal steroids (to decrease overall prematurity-associated neonatal morbidities), continuous fetal heart rate monitoring and delivery if/when warranted.34,44 Specifically, we do not hesitate to deliver in prematurity (preferably after IM antenatal steroids) if/when indicated with the premise that preterm delivery is preferable to stillbirth, neonatal compromise at delivery and potential life-long sequelae of hypoxia/acidosis at delivery.
Governing Body Guidelines
The PRACTICE PARAMETER for the Performance of Standard Diagnostic Obstetrical Ultrasound of the American College of Radiology (ACR), American College of Obstetricians and Gynecologists (ACOG), Society of Maternal Fetal Medicine (SMFM), Society of Radiology of Ultrasound (SRU), ACR-ACOG-AIUM-SMFM-SRU, regarding the umbilical cord published in 2018, simply states: “The umbilical cord should be imaged and the number of vessels in the cord documented. The placental cord insertion site should be documented when technically possible”.54 In a similar fashion, the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG), does not address the topic of nuchal, true knots of the umbilical cord or complex umbilical cord entanglement.55 Specific MR imaging criteria for utilization for a detailed depiction of umbilical cord entanglement (nuchal, true knots and complex entanglement) remain to be determined.56 Current ACOG directives for indicated preterm birth fail to address the potential implications of complex umbilical cord entanglement, or for that matter, the true knot of the umbilical cord (namely stillbirth) and do hence do not include and the possible option of early preterm delivery in this group of patients.57 In our opinion, despite the relative rarity of this diagnosis, absent directives constitute an omission, which requires amendment.
Summary
Respectfully, we believe that following a prenatal sonographic diagnosis of complex umbilical cord entanglement, it is both morally and ethically incorrect to withhold this information from the patient. It is our opinion that complete disclosure of complex umbilical cord entanglement should be conducted with the patient in real-time upon sonographic diagnosis. This counseling should be based upon available case-specific sonographic findings, and include the possibility of false-positive findings (if/when appropriate), the importance of attention to fetal movements, planned frequent intermittent fetal testing, and documented. Furthermore, we believe that in cases of complex umbilical cord entanglement that unless warranted earlier, consideration should be given to early-term delivery (induction of labor unless otherwise indicated) ≥37 weeks’ gestation even in the absence of fetal compromise in an effort to negate the potential of stillbirth during the remaining 4 weeks of gestation. Thus, the option for early-term delivery should be left to the discretion of the patient and her physician. The above-outlined management we recommend (although at a considerably later gestational age) would be akin to the ACOG guidelines for the considerably earlier delivery of monochorionic monoamniotic twins between 32 and 34 and 6/7 weeks’ gestation, secondary to umbilical cord entanglement omnipresent in these rare pregnancies (Figures 6–8), and the associated increased risk of stillbirth due to umbilical cord entanglement.54
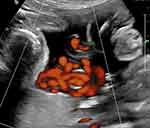 |
Figure 6 Power Doppler imaging of complex umbilical cord entanglement in monochorionic monoamniotic twins at 27 weeks’ gestation. |
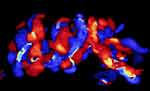 |
Figure 7 Three dimension (3D) color Doppler imaging of complex umbilical cord entanglement in monochorionic monoamniotic twins at 27 weeks’ gestation. |
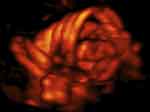 |
Figure 8 Three dimension power (3D) Doppler imaging of complex umbilical cord entanglement in monochorionic monoamniotic twins at 27 weeks’ gestation. The monoamniotic twins depicted in Figure 6–8 were delivered by emergency Cesarean at 29 and 1/7 weeks’ gestation, due to prolonged fetal bradycardia of twin B (60 bpm for 14 minutes), one day after admission for continuous fetal heart rate monitoring and intramuscular rescue steroids to decrease overall prematurity-associated neonatal morbidities should premature delivery become indicated. At delivery, marked entanglement of the umbilical cords and complex umbilical cord entanglement of twin B (a true knot and nuchal cord), were noted. Birth weights were 1195 and 1385 grams, respectively; Apgar scores were 5/7/9 and 6/8/9 at 1, 5 and 10 minutes, respectively, and umbilical artery gas analyses; pH = 7.27 base excess = −3.4 and 7.22 and −4.6, for twins A and B, respectively. Both infants did well. |
Accordingly, we suggest that consideration be given to amending current governing body guidelines for the timing of delivery to reflect such occurrences, thus providing patients with prenatal sonographic diagnosis of complex umbilical cord entanglement the option of early-term delivery ≥37 weeks’ gestation, if not clinically indicated earlier.57
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu LC, Huang HB, Yu MH, Su HY. Analysis of intrauterine fetal demise – a hospital-based study in Taiwan over a decade. Taiwan J Obstet Gynecol. 2013;52(4):546–550. doi:10.1016/j.tjog.2013.10.016
2. Lehtonen T, Marrkula T, Soidinsalo P, Otonkonski S, Laine J. Causes of stillbirth in Turku, Finland, 2001–2011. Pediatr Dev Pathol. 2017;20(1):5–15. doi:10.1177/1093526616686236
3. Hammad IA, Blue NR, Allhouse AA, et al. NICHD stillbirth collaborative research network group. Umbilical cord abnormalities and stillbirth. Obstet Gynecol. 2020;135(3):644–652. doi:10.1097/AOG.0000000000003676
4. Parast MM, Crum CP, Boyd TK. Placental histologic criteria for umbilical blood flow restriction in unexplained stillbirth. Hum Pathol. 2008;39(6):948–953. doi:10.1016/j.humpath.2007.10.032
5. Ryan ED, Trivedi N, Bernirschke K, Lacoursiere DY, Parast MM. Placental histologic criteria for diagnosis of cord accident: sensitivity and specificity. Pediatr Dev Pathol. 2012;15(4):275–280. doi:10.2350/11-12-1127-OA.1
6. Benirschke K, Kaufman P. Anatomy and pathology of the umbilical cord and major fetal vessels. In: Benirschke K, Kaufman P, editors. Pathology of the Human Placenta.
7. Raio L, Ghezzi F, Di Naro E, Franchi M, Bruhwiler H. Prenatal assessment of the Hyrtl anastomosis and evaluation of its function: case report. Hum Reprod. 1999;14(7):1890–1893. doi:10.1093/humrep/14.7.1890
8. Raio L, Ghezzi F, Di Naro E, Balestrei D, Duria P, Scheinder H. In-utero characterization of the blood flow in Hyrtl anastomosis. Placenta. 2001;22(6):597–601. doi:10.1053/plac.2001.0685
9. Nkwabong E, Ndoumbe Mballo J, Dobbit JS. Risk factors for nuchal cord entanglement at delivery. Int J Gynaecol Obstet. 2018;141(1):108–112. doi:10.1002/ijgo.12421
10. Ogueh O, Al-Tarkait A, Vallerand D, et al. Obstetrical factors related to nuchal cord. Acta Obstet Gynecol Scand. 2006;85(7):810–814. doi:10.1080/00016340500345428
11. Rhoades DA, Latza U, Mueller BA. Risk factors and outcomes associated with nuchal cord. A population-based study. J Reprod Med. 1999;44(1):39–45.
12. Dursun P, Salman MC, Ozyuncu O, Aksu T. Nuchal cord type B associated with an excessively long umbilical cord as a cause of stillbirth. Clin Exp Obstet Gynecol. 2004;31:58–59.
13. Spellacy WN, Graven H, Fisch RO. The umbilical cord and complications of true knot, nuchal cords, and cords around the body. Am J Obstet Gynecol. 1966;94(8):1136–1142. doi:10.1016/0002-9378(66)90777-0
14. Larson JD, Rayburn WF, Harlan VL. Nuchal cord entanglements and gestational age. Am J Perinatol. 1997;14(09):555–557. doi:10.1055/s-2007-994333
15. Clapp JF, Stepanchak W, Hashimoto K, Ehrenberg H, Lopez B. The natural history of nuchal cords. Am J Obstet Gynecol. 2003;189(2):488–493. doi:10.1067/S0002-9378(03)00371-5
16. Schäffer L, Burkhardt T, Zimmermann R, Kurmanavicius J. Nuchal cords in term and postterm deliveries – do we need to know? Obstet Gynecol. 2005;106(1):23–28. doi:10.1097/01.AOG.0000165322.42051.0f
17. Crawford JS. Cord around the neck: incidence and sequelae. Acta Paediatr. 1962;51(5):594–603. doi:10.1111/j.1651-2227.1962.tb06586.x
18. Crawford JS. Cord around the neck: further analysis of incidence. Acta Paediatr. 1964;53(6):553–557. doi:10.1111/j.1651-2227.1964.tb07267.x
19. Shui KP, Eastman NJ. Coiling of the umbilical cord around the fetal neck. J Obstet Gynaecol Br Emp. 1957;64(2):227–228. doi:10.1111/j.1471-0528.1957.tb02625.x
20. Dipple AL. Maligned umbilical cord entanglements. Am J Obstet Gynecol. 1964;88(8):1012–1019. doi:10.1016/S0002-9378(16)35085-2
21. Hinkson L, Pahlitzsch T, Henrich W. Sextuple rings of nuchal cord by breech presentation: a warning sign. Ultrasound Obstet Gynecol. 2019;54(6):843–844. doi:10.1002/uog.20376
22. Judy C, Bell LA. A case of seven nuchal loops and a review of the literature. Mo Med. 2005;102(6):569–570.
23. Wang Y, Le Ray C, Audibert F, Wagner MS. Management of nuchal cord with multiple loops. Obstet Gynecol. 2008;112(2, Part 2):460–461. doi:10.1097/AOG.0b013e31816fd75c
24. Dodds M, Windrim R, Kingdom J. Complex cord entanglement. J Matern Fetal Neonatal Med. 2012;25(9):1827–1829. doi:10.3109/14767058.2011.644361
25. Duffy S, Cochrane R. A difficult delivery associated with a nuchal cord found nine times around the fetal neck. J Obstet Gynaecol. 2007;27(8):859–860. doi:10.1080/01443610701748336
26. Mian DB, Konan J, Kouakou KC, Angoi V, Gbary E, Itoua C. Severe antenatal strangulation and sudden fetal death occurs at term. Clin Exp Obstet Gynecol. 2016;43(1):161–164.
27. Ranzini AC, Walters CA, Vintzileos AM. Ultrasound diagnosis of nuchal cord; the gray-scale divot sign. Obstet Gynecol. 1999;93(Pt 2):854.
28. Jauniaux E, Mawissa C, Peelaerts C, Rodesch F. Nuchal cord in normal third-trimester pregnancy: a color Doppler imaging study. Ultrasound Obstet Gynecol. 1992;2(6):417–419. doi:10.1046/j.1469-0705.1992.02060417.x
29. Morgan-Ortiz F, Rodriguez-Ontiveros C, Chang-Batiz H, Avila-Vergara MA. Evaluation of ultrasound as a diagnostic test in nuchal encirclement by the umbilical cord during labor. Ginecol Obstet Mex. 1997;65:529–532.
30. Qin Y, Wang CC, Lau TK, Rogers MS. Color sonography: a useful technique in the identification of nuchal cord during labor. Ultrasound Obstet Gynecol. 2000;15(5):413–417. doi:10.1046/j.1469-0705.2000.00113.x
31. Romero-Gutierrez G, Estrada Razo S, Chavez Curiel A, Ponce Ponce de Leon AL. Color flowmetry values in fetuses with nuchal cord encirclement. Ginecol Obstet Mex. 2000;68:401–407.
32. Ramòn y Cajal CL, Martinez RO. Prenatal diagnosis of a true knot of the umbilical cord. Ultrasound Obstet Gynecol. 2004;23:99–100.
33. Hasbun J, Alacalde JL, Sepulveda W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: values and limitations. J Ultrasound Med. 2007;26(9):1215–1220. doi:10.7863/jum.2007.26.9.1215
34. Sherer DM, Dalloul M, Ward K, et al. Coexisting true umbilical cord knot and nuchal cord: possible cumulative increased risk of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2017;50(3):404–405. doi:10.1002/uog.17389
35. Miser WF. Outcome of infants born with nuchal cords. J Family Prac. 1992;34:44–45.
36. Mastrobattista JM, Hollier LM, Yeomans ER, et al. Effects of nuchal cord on birthweight and immediate neonatal outcomes. Am J Perinatol. 2005;22(02):83–85. doi:10.1055/s-2005-837737
37. Jauniaux E, Ramsay B, Peellaerts C, Scholler Y. Perinatal features of pregnancies complicated by nuchal cord. Am J Perinatol. 1995;12(4):255–258. doi:10.1055/s-2007-994467
38. Schreiber H, Daykan Y, Arbib N, Markovitch O, Berkovitz A, Biron-Shental T. Adverse pregnancy outcomes and multiple nuchal cord loops. Arch Gynecol Obstet. 2019;300(2):279–283. doi:10.1007/s00404-019-05178-w
39. Mariya T, Fujibe Y, Shinkai S, et al. Multiple part cord entanglement and neonatal outcomes. Taiwan J Obstet Gynecol. 2018;57(5):672–676. doi:10.1016/j.tjog.2018.09.001
40. Önderglu LS, Dursun P, Durukan T. Perinatal features and umbilical cord blood gases in newborns complicated with nuchal cord. Turk J Pediatr. 2008;5:466–470.
41. Hoh JK, Sung YM, Park MI. Fetal heart rate parameters and perinatal outcome in fetuses with nuchal cords. J Obstet Gynaecol Res. 2012;38(2):358–363. doi:10.1111/j.1447-0756.2011.01707.x
42. Sepulveda W. Antenatal course and perinatal outcome after ultrasound detection of triple nuchal cord: a case series. J Matern Fetal Neonatal Med. 2019;16:1–6.
43. Gurau D, Zaltz A, Yoo WK, Rahmani MR. All tied up and nowhere to go: report of a figure-eight umbilical cord complex true knot and triple nuchal cord detected on antenatal sonography. J Ultrasound Med. 2016;35(6):1361–1363. doi:10.7863/ultra.15.09044
44. Sherer DM, Khoury-Collado F, Dalloul M, et al. Recurrent antepartum compression of a single artery double nuchal cord necessitating emergency Cesarean delivery. Am J Perinatol. 2005;22(08):437–440. doi:10.1055/s-2005-916286
45. Bord A, Yagel S, Valsky D. Overburdened and undernourished. Am J Obstet Gynecol. 2007;197(3):
46. Sherer DM, Dalloul M, Sabir S, London V, Haughton M, Abulafia O. Persistent quadruple nuchal cord throughout the third trimester associated with decelerating fetal growth. Ultrasound Obstet Gynecol. 2017;49(3):409–410. doi:10.1002/uog.15940
47. Zbeidy R, Souki FG. One long cord, four nuchal loops and a true knot. BMJ Case Rep. 2017;2017:bcr2017223241. doi:10.1136/bcr-2017-223241
48. Sherer DM, Manning FA. Prenatal ultrasonographic diagnosis of nuchal cord(s): disregard, inform, monitor, or intervene? Ultrasound Obstet Gynecol. 1999;14(1):1–8. doi:10.1046/j.1469-0705.1999.14010001.x
49. Sherer DM, Amoabeng O, Dryer AM, Dalloul M. Current perspectives of prenatal sonographic diagnosis and clinical management challenges of true knot of the umbilical cord. Int J Womens Health. 2020;12:221–233. doi:10.2147/IJWH.S192260
50. Sherer DM, Ward K, Bennett M, Dalloul M. Current perspectives of prenatal sonographic diagnosis and clinical management challenges of nuchal cord(s). Int J Womens Health. 2020;12:623–631. doi:10.2147/IJWH.S211124
51. Bohiltea RE, Turcan N, Cirstoiu M, Shukla RC. Prenatal ultrasound diagnosis of umbilical cord knot – debate regarding ethical aspects of a series of cases. J Med Life. 2016;9(3):297–301.
52. Vasij O, Matijevic R, Blagaic V, Mislovic B. Do we sometimes see too much? Prenatal diagnosis of a true umbilical cord knot. Eur J Obstet Gynecol Reprod Biol. 2015;187:73–74. doi:10.1016/j.ejogrb.2015.02.023
53. Stempel LE. Beyond the pretty pictures. Giving obstetricians just enough (umbilical) cord to hang themselves. Am J Obstet Gynecol. 2006;195(4):888–890. doi:10.1016/j.ajog2006.06.001
54. AIUM-ACR-ACOG-SMFM-SRU practice parameter for the performance of standard diagnostic obstetrical ultrasound examinations. J Ultrasound Med. 2018;9999:12. doi:10.1002/jum.14831
55. Salomon LJ, Alfirevic Z, Berghella V, et al. on behalf of the ISUOG clinical standards committee. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2011;37(1):116–126. doi:10.1002/uog.8831
56. Kumar I, Verma A, Jain M, Shukla RC. Structured evaluation and reporting in imaging of placenta and umbilical cord. Acta Radiol. 2020;61(5):685–704. doi:10.1177/0284185119875644
57. Gyamfi-Bannerman C, Gantt AB, Miller RS and the Society for Maternal-Fetal Medicine. Medically indicated late-preterm and early term deliveries. ACOG Committee Opinion Summary Number. 818. Obstet Gynecol. 2021;137(2):388–391. doi:10.1097/AOG.0000000000004245.. doi:10.1097/01.AOG.0000428648.75548.00
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.