Back to Journals » International Medical Case Reports Journal » Volume 16
19 Months Toddler with a Giant Oral Capillary Hemangioma, a Case Report
Authors Kabagenyi F , Anena SP , Seguya A
Received 2 February 2023
Accepted for publication 9 May 2023
Published 15 May 2023 Volume 2023:16 Pages 287—291
DOI https://doi.org/10.2147/IMCRJ.S406901
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Fiona Kabagenyi,1 Sandra Petti Anena,1 Amina Seguya2
1Department of Ear Nose and Throat, Makerere University, Kampala, Uganda; 2Department of Ear Nose and Throat, Mulago National Referral Hospital, Kampala, Uganda
Correspondence: Fiona Kabagenyi, Department of Ear Nose and Throat, College of Health Sciences, Makerere University, P.O. Box 7072, Kampala, Uganda, Tel +256774150102, Email [email protected]
Abstract: Head and neck vascular tumors are common in children. Capillary hemangiomas are often easily confused with pyogenic granulomas due to histopathological resemblance. Furthermore, predisposing factors to pyogenic granulomas include an existing hemangioma, which may be co-existing entities. Surgical excision of large unsightly tumors causing functional deficits is a feasible management option. We report a case of a rapidly growing oral lesion in a toddler with feeding difficulties and anemia. It triggered a diagnostic dilemma as it was clinically consistent with a pyogenic granuloma but histologically diagnosed as a capillary hemangioma. It was successfully excised with no recurrence after 6 months.
Keywords: hemangioma, pyogenic granuloma, head and neck vascular tumours
Introduction
The International Society for the study of Vascular Anomalies (ISSVA) classifies vascular lesions into vascular malformations and vascular tumors.1–3 Vascular tumors include congenital hemangiomas, infantile hemangiomas, and pyogenic granulomas.4 Hemangiomas are further pathologically classified as capillary, cavernous, or mixed hemangiomas.5,6 Unique clinical characteristics exist across the differential diagnoses of vascular tumors.7 However, histopathological resemblance between capillary hemangioma and pyogenic granuloma may cause diagnostic challenges. Fifty-six percent of lesions classified as capillary hemangiomas were found to be true pyogenic granulomas.8 In fact, the synonym for pyogenic granuloma is a lobular capillary hemangioma.9 This further complicates the tailored management of vascular tumors. Clinical judgment to differentiate between the two entities may contribute to initial management option. Although both capillary hemangiomas and pyogenic granulomas are common lesions in the head and neck region, which can affect the oral cavity,9,10 their natural history and predisposing factors differ.2,4,7,11 Capillary hemangioma appears gradually within the first year of life2 while the pyogenic granuloma typically appears rapidly after 1 year of life.4 Predisposing factors of capillary hemangioma include being female and Caucasian and born with low birth weight6 while those of pyogenic granulomas include having hemangiomas, dermatologic eczematous lesions, and trauma.12 These should be looked out for. Noteworthy, 77% of those with pyogenic granulomas may have no predisposing factor.11 Examination of congenital hemangiomas may show expansion in color alteration on crying, which is not the case in pyogenic granulomas.7
Variable treatment options such as watchful monitoring, pharmacological and surgical options are given in both capillary hemangiomas and pyogenic granulomas.12–15 Surgical management is indicated for lesions causing functional deficits and causing complications such as ulcers and bleeding, periorbital sight threatening lesions, airway obstructive lesions, and threat to cosmesis.12–15 A 0–5% recurrence rate is reported following excision of pyogenic granulomas.12,13,16
We present an interesting case of a young girl with a rapidly growing oral mass originating in the left floor of mouth, which was referred to our Paediatric Ear, Nose, and Throat (ENT) clinic from the Paediatric Oncology Department at Mulago National Referral Hospital. To the best of our knowledge, this is the first report in the literature of a giant capillary hemangioma arising from the floor of the mouth and postulated to cause anemia.
Case Report
A 19-month-old female, otherwise healthy, presented with a month’s history of an oral mass. Her mother noticed a small oral mass that started growing from the left underside of the tongue. Initially, it did not affect her speech or feeding. She was given antibiotics from drug shops during the first fortnight with minimal improvement. In the next 2 weeks, the mass rapidly increased in size, covering more than two-thirds of her mouth, pushing the tongue to the side. She could no longer close her mouth, had trouble articulating words and was drooling all the time. However, no change in voice, stridor, or difficulty in breathing were reported. Due to her continued struggle to feed, her mother resorted to frequent pureed feeds. No choking or coughing episodes on feeds were reported. The mass was smelly, had a whitish coating with occasional contact bleeding. Save for the open mouth, she slept well. There was no history of fever, trauma, or other swellings or discolorations on the rest of the body or bleeding tendencies. Other medical, surgical, and family history were unremarkable.
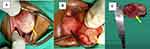
On clinical exam, she was well-nourished, afebrile but very irritable. She had facial symmetry with no stigmata for any syndrome. She had a normal cry, with moderate anterior drooling and a large oral mass (5*4*3 cm) arising from the left floor of mouth, which occluded three-quarters of her mouth (Figure 1A). It was a firm, non-fluctuant, oval mass coated with whitish debris on the surface and a pink to purple discoloration on the undersurface. Her tongue was mobile, free from the mass, and pushed to the right corner of the mouth. No petechiae or ulcers were seen. The ears, nose, neck, and other systemic examinations were normal.
After obtaining informed consent for an excisional biopsy, preoperative blood work up and imaging were done. Serum electrolytes, leukocytes and platelet counts were within normal ranges, but the hemoglobin level was 7 g/dl. Blood was therefore booked for intraoperative and post-operative transfusion after grouping and cross matching. The COVID-19 PCR was negative. A contrasted Computerized Tomography (CT) scan of the head and neck showed a regular mass in the left oral cavity that took up contrast. Fat planes in floor of mouth and tongue were intact. Preoperative Paediatric Anesthesiologist’s review was done for airway management plan.
Intraoperatively, successful nasal intubation was performed. The easily friable lesion had a sessile base extending from 1 cm posterior to the frenulum, to the retro-molar trigone on the left with serpentine vascular vessels across the left floor of mouth (Figure 1B). The rest of the oral cavity was normal.
The left lingual nerve and left submandibular duct were identified and spared. The lesion was completely enucleated (Figure 1C). Hemostasis was achieved with bipolar cautery and ligature ties. Blood loss was minimal. The defect in the floor of mouth was closed using interrupted absorbable sutures.
Post-operatively, the child had blood transfusion with packed cells, oral antibiotics, analgesia, and oral care with saline rinses. She had mild hypoglossal paresis when she woke up but was able to feed with a graded diet 2-h post-operatively. She had an uneventful 24-h observation and was discharged the following day.
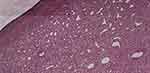
Histology showed a diagnosis of capillary hemangioma secondarily infected (Figure 2). GLUT 1 testing is not locally available and thus was not done.
Follow-up at 2 weeks showed complete resolution of symptoms with normal tongue movement, and at 6 months she had normal oral cavity mucosa and tongue movement (Figure 3).
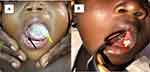 |
Figure 3 Pre-surgical view (A) Shows tongue pushed to the right (black arrow) by the oral lesion (yellow arrow). The post-surgical view (B) 6 months later shows healed floor of mouth (red arrow). |
The child’s parent provided informed consent for the case details and any accompanying images to be published, available upon request. Institutional approval was not required to publish the case details.
Discussion
Oral hemangiomas have been reported to include the gingiva,17 tongue,18–20 and hard palate,21–23 and to present more in females.6,24,25 This is similar to our case of a female child, with a lesion arising from the floor of mouth. There were no known predisposing factors to either capillary hemangioma or pyogenic granuloma. The clinical onset after 1 year of life, rapid growth, and tendency to bleed raised our suspicion of a pyogenic granuloma. To our surprise, histology came back as an infected capillary hemangioma.
Paglial et al in their study of 128 children found that 5% of children with pyogenic granulomas had hemangiomas. Their average lesion size was 7mm, presenting for about 5 months on average with a 1–2-month delay before treatment.12 Our case did not seem to have a hemangioma before the current presentation, was more than 50mm in size, and progressed over a month with significant functional deficits, enough to render urgent surgical intervention. It is postulated to have caused significant anemia and required blood transfusion. Large lesions in hemangiomas that are greater than 10cm2 may lead to a 4% increase in the requirement for intervention and 5% increase in complication.26 Indications for surgery, though rare, include presence of ocular, airway, auditory, feeding complications, cosmetic problems, or even congestive cardiac failure.2 Our case had significant difficulty in feeding with occasional bleeding, and frequent drooling that was unsightly.
Paglial et al found that shave excision and electrocautery were the most common treatment methods for pyogenic granulomas, followed by laser therapy. Other methods included punch excision, liquid nitrogen, and observation. Regression of the pyogenic granulomas was seen in 4.5% of patients that had no treatment, over 6–18 months.12 In a retrospective study, 80% of 408 patients with pyogenic granulomas had surgical complete excision, 19% were treated by curettage, shave excision, or cautery or a combination of these. The overall recurrence rate was 5% with shave excision, curettage, and cautery having a recurrence rate of 10% compared with 3.6% following excision and closure.13 Due to the large size of the lesion, complete surgical excision with electrocautery was used with no recurrence seen in our case.
Histology remains the mainstay of diagnosis of vascular tumors. In one study where most cases were classified as capillary hemangiomas, 56% were in fact pyogenic granulomas.8 Another study utilizing GLUT-1 as a biomarker found that 34% of 77 biopsies for oral hemangiomas were true hemangiomas.9 Unfortunately, this biomarker is not locally available and thus was not done in our case, and our quest for answers remains. Luckily, this does not prognosticate clinical outcome. Biannual outpatient reviews will monitor any interval changes going forward.
Conclusion
Although vascular lesions of the oral cavity in children can be successfully observed, fast growing lesions that cause functional impairment and anemia should be considered for prompt surgical management to prevent further complications. Clinical correlation with histological findings is key in treatment planning.
Acknowledgments
We acknowledge the interdisciplinary team (Paediatric Oncology, Paediatric Anesthesiology, and Paediatric ENT) that was involved in the care of this patient. We also acknowledge parental consent to sharing this rare case with the scientific community.
Author Contributions
All authors made a significant contribution to the work reported in all these areas; conception, drafting, revising, and critically reviewing the article; agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no financial interest or other competing interest.
References
1. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69(3):412–422.
2. Gampper TJ, Morgan RF. Vascular anomalies: hemangiomas. Plast Reconstr Surg. 2002;110(2):572–585. doi:10.1097/00006534-200208000-00032
3. Elias G, McMillan K, Monaghan A. Vascular lesions of the head and oral cavity – diagnosis and management. Dent Update. 2016;43(9):859–60, 62–4, 66. doi:10.12968/denu.2016.43.9.859
4. Barrón-Peña A, Martínez-Borras MA, Benítez-Cárdenas O, Pozos-Guillén A, Garrocho-Rangel A. Management of the oral hemangiomas in infants and children: scoping review. Med Oral Patol Oral Cir Bucal. 2020;25(2):e252–e61. doi:10.4317/medoral.23329
5. Batsakis J. Tumours of the Head and Neck.
6. Covelli E, Seta ED, Zardo F, Seta DD, Filipo R. Cavernous hemangioma of the external ear canal. J Laryngol Otol. 2008;19:1–3.
7. Wadhwani M, Singh R. Capillary hemangioma–a review. Clin Exp Vis Eye Res. 2020;3(1):13. doi:10.15713/ins.clever.41
8. Iwata J, Sonobe H, Furihata M, Ido E, Ohtsuki Y. High frequency of apoptosis in infantile capillary haemangioma. J Pathol. 1996;179(4):403–408. doi:10.1002/(SICI)1096-9896(199608)179:4<403::AID-PATH604>3.0.CO;2-E
9. da Silva WB, Ribeiro ALR, de Menezes SAF, de Jesus Viana Pinheiro J, de Melo Alves-Junior S. Oral capillary hemangioma: a clinical protocol of diagnosis and treatment in adults. Oral Maxillofac Surg. 2014;18(4):431–437. doi:10.1007/s10006-013-0436-z
10. Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021; 2021.
11. Lian T. Benign tumors and tumor-like lesions of the oral cavity. In: Cummings Otolaryngology-Head and Neck Surgery. Elsevier mosby; 2005:1574.
12. Pagliai KA, Cohen BA. Pyogenic granuloma in children. Pediatr Dermatol. 2004;21(1):10–13. doi:10.1111/j.0736-8046.2004.21102.x
13. Giblin A, Clover A, Athanassopoulos A, Budny P. Pyogenic granuloma–the quest for optimum treatment: audit of treatment of 408 cases. J Plast Reconstr Aesthet Surg. 2007;60(9):1030–1035. doi:10.1016/j.bjps.2006.10.018
14. Ethunandan M, Mellor TK. Haemangiomas and vascular malformations of the maxillofacial region—a review. Br J Oral Maxillofac Surg. 2006;44(4):263–272. doi:10.1016/j.bjoms.2005.06.032
15. Osifo DO, Evbuomwan I. Hemangiomas in children: challenges and outcome of surgical management in Benin city, Nigeria. Iran J Pediatr. 2011;21(3):350–356.
16. Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8(4):267–276. doi:10.1111/j.1525-1470.1991.tb00931.x
17. Mishra MB, Bishen KA, Yadav A. Capillary hemangioma: an occasional growth of attached gingiva. J Indian Soc Periodontol. 2012;16(4):592–596. doi:10.4103/0972-124X.106924
18. Peters SM, Koslovsky DA, Yoon AJ, Philipone EM. Pyogenic granuloma in the tongue in a five-year-old: a case report. J Clin Pediatr Dent. 2018;42(5):383–385. doi:10.17796/1053-4625-42.5.10
19. Akyol MU, Yalçiner EG, Doğan AI. Pyogenic granuloma (lobular capillary hemangioma) of the tongue. Int J Pediatr Otorhinolaryngol. 2001;58(3):239–241. doi:10.1016/S0165-5876(01)00425-6
20. Zielnik Jurkiewicz B. Rzadki przypadek ziarniniaka ropotwórczego jezyka u 8-tygodniowego niemowlecia [Rare case of pyogenic granuloma of the tongue in an 8-week-old child]. Otolaryngol Pol. 2005;59(5):755–758. Polish.
21. Bakshi J, Virk RS, Verma M. Pyogenic granuloma of the hard palate: a case report and review of the literature. Ear Nose Throat J. 2009;88(9):E4–E5.
22. Costello L, Huston M. An unusual presentation of a pyogenic granuloma in a paediatric patient. J Paediatr Child Health. 2019;55(4):486–487. doi:10.1111/jpc.14415
23. Havle AD, Shedge SA, Dalvi RG. Lobular capillary hemangioma of the palate -a case report. Iran J Otorhinolaryngol. 2019;31(107):399–402. doi:10.22038/ijorl.2019.38928.2285
24. Tangtatco JA, Freedman C, Phillips J, Pope E. Surgical treatment outcomes of infantile hemangioma in children: does prior medical treatment matter. Pediatr Dermatol. 2018;35(6):e418–e419. doi:10.1111/pde.13658
25. Coloma CB, Martínez IM, Carrió CP, Gil SG, Diago MP, Sanz JM. Clinical characteristics, treatment and outcome of 28 oral haemangiomas in paediatric patients. Med Oral Patol Oral Cir Bucal. 2011;16(1):5.
26. Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118(3):882–887. doi:10.1542/peds.2006-0413
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.