Back to Journals » OncoTargets and Therapy » Volume 13
125I Seed Brachytherapy Combined with Single-Agent Chemotherapy in the Treatment of Non-Small-Cell Lung Cancer in the Elderly: A Valuable Solution
Received 20 July 2020
Accepted for publication 8 September 2020
Published 16 October 2020 Volume 2020:13 Pages 10581—10591
DOI https://doi.org/10.2147/OTT.S272898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Tian-Hua Yue,1,2 Wei Xing1
1Medical Imaging Department, The Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu 213000, People’s Republic of China; 2Department of Interventional Radiology, The Affiliated Jianhu Hospital of Nantong University, Jiangsu, Jianhu 224700, People’s Republic of China
Correspondence: Wei Xing
Medical Imaging Department, The Third Affiliated Hospital of Soochow University, 185 Juqian Road, Changzhou, Jiangsu 213000, People’s Republic of China
Tel +86 13961236568
Email [email protected]
Purpose: The aim of this study was to compare the effectiveness and safety of CT-guided 125I seed brachytherapy combined with single-agent chemotherapy versus combined chemotherapy in the treatment of elderly NSCLC.
Materials and Methods: We retrospectively analyzed 110 patients (64 men and 46 women; mean age=71.25± 7.14 years) who were diagnosed with NSCLC without distant metastases between January 2015 and May 2020. A total of 50 patients received 125I brachytherapy combined with single-agent chemotherapy (group A), whereas 60 patients received combined chemotherapy (group B). The response to therapy and adverse effect were compared between groups. The local response rate was evaluated by CT. Progression-free survival (PFS) and overall survival (OS) data were obtained through clinical follow-up.
Results: All patients had been treated and were followed-up for 3– 60 months. The median OS and PFS were 23.71± 1.41 months (95% CI=20.95– 26.47) vs 16.12± 0.93 months (95% CI=14.31– 17.93) (P< 0.05) and 15.08± 0.85 months (95% CI=13.42– 16.74) vs 10.03± 0.53 months (95% CI=9.01– 11.06) (P< 0.05) in group A and group B, respectively. The local response rate and clinical symptoms of patients in group A were significantly relieved when compared with group B. Severe complications were not observed in either group.
Conclusion: CT-guided 125I seed brachytherapy combined with single-agent chemotherapy is an effective and safe therapy and shows promising results compared to combined chemotherapy alone for NSCLC in the elderly. A randomized study will be needed to assess the superiority of this combined modality treatment.
Keywords: 125I seed, brachytherapy, chemotherapy, elderly, NSCLC
Introduction
Lung cancer is one of the most common malignancies and the leading cause of cancer death worldwide, most of which is non-small-cell lung cancer (NSCLC).1–3 Due to the intensification of population aging, rural urbanization, and industrialization of cities and towns, the environment pollution, unhealthy lifestyle, smoking, and other factors, lung cancer has a high morbidity and mortality in China.4 Currently, surgical resection, chemotherapy, and radiotherapy are the standard treatment methods for NSCLC, all of which have defects. Surgical resection has been the preferred treatment for the management of early-stage NSCLC (ie, clinical stages I and II), but the reported 5-year survival rate is 50–70% after surgical resection.5,6 Some patients with early-stage NSCLC are not suitable for the surgery, because of the special site of NSCLC, such as being close to the pulmonary hilus or heart. Likewise, some patients with poor general condition make them intolerant to anesthesia and surgery. Patients with early-stage NSCLC treated with radiofrequency ablation experienced a significant increase in overall survival.7 However, Iguchi et al8 suggested that the 5-year overall and progression-free survival rates were 58.9% and 39.9%, respectively, for radiofrequency ablation; 85.5% and 75.9%, respectively, for sublobar resection in patients with clinical stage I NSCLC. Recently, the emerging molecular targeting therapy has helped to control lung cancer, but its long-term survival rate is limited and expensive.9 Gomes and Cruz10 showed the mechanism of resistance to targeted therapy, such as secondary mutations in the EGFR gene, amplification of Human Epidermal Growth Factor Receptor 2 (HER2) gene, mutations in PIK3CA and BRAF, and conversion to small-cell lung cancer. Therefore, external radiotherapy combined with chemotherapy as the basic treatment of malignant tumors has played a major role in the management of patients with non-surgical lung cancer.11,12 A lot of clinical studies have confirmed the effectiveness of chemotherapy combined with external radiotherapy in the treatment of NSCLC, which could prolong survival time and improve the quality-of-life of patients.13,14 However, many patients (especially older patients) cannot tolerate the currently available treatment modalities due to their poor general condition (eg, chronic obstructive emphysema, poor cardio-pulmonary function, hypertension, diabetes, and other diseases), severe toxicity after chemotherapy, and external radiotherapy (such as myelosuppression, nausea, radiation pneumonitis, radiation esophagitis, etc.), even if using the latest technology. A study for unfit elderly patients with NSCLC indicated that single-agent chemotherapy should be considered the standard treatment.15 Meanwhile, owing to their poor general condition and other factors, 125I seed brachytherapy may be an alternative method for elderly NSCLC.16
125I seed brachytherapy has been applied in clinical practice and achieved a good outcome for several years. Studies have shown that 125I seed brachytherapy is a safe and useful minimally invasive therapy for tumors (pancreatic cancer, liver cancer, gynecologic malignancies and brain cancer, etc.).9,–17–22 Many researchers have begun to explore the 125I seed brachytherapy treatment for NSCLC. The results have showed that percutaneous pulmonary 125I seed brachytherapy can control local tumors well, while not increasing other serious complications.6 Now, 125I seed brachytherapy, which is a form of internal radiotherapy, has played an important role in NSCLC. The average energy of 125I seed is 27–32 KeV, which releases low doses of γ-rays and soft X-rays continuously. 125I seed had an initial activity of 0.8 mCi, a half-life of 59.6 days and a radiation radius of 1.7 cm with a total dose administration of approximately 110–160 Gy. Because the radiation energy from 125I seed decreases with increasing distance, there is a much lower dose to normal neighboring organs. Therefore, computed tomography (CT)-guided 125I seed brachytherapy can target the entire dose irradiation to the local tumor and there are no obvious radiation-related adverse reactions, which is especially suitable for elderly NSCLC.
Thus, we considered the possibility that 125I seed brachytherapy combined with single-agent chemotherapy might be an alternative treatment for elderly NSCLC. The purpose of this study is to evaluate the effectiveness and safety of CT-guided 125I seed brachytherapy combined with single-agent chemotherapy in the treatment of elderly NSCLC.
Materials and Methods
Patient Selection
Between January 2015 and May 2020, 110 patients (64 men and 46 women; mean age=71.25±7.14 years) who were diagnosed with NSCLC without distant metastases at our hospital were enrolled for this retrospective study. Then, 50 patients (group A) received percutaneous CT-guided 125I seed brachytherapy and single-agent chemotherapy, and 60 patients (group B) were given platinum-based combination chemotherapy. This retrospective study was approved by the institutional review board at our hospital according to the standards of the Declaration of Helsinki. The ethics committee of our hospital deemed some patients consent not necessary due to the retrospective nature of this study. But the other patients provided written informed consent. The data of all patients was anonymized and deidentified prior to analysis in this study. The characteristics of individual patients are summarized in Table 1. The TNM staging of the tumors used the American Joint Committee on Cancer eighth Edition.
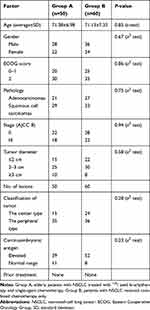 |
Table 1 Summary of Patient and Tumor Characteristics |
Inclusion and Exclusion Criteria
Study inclusion criteria were determined as follows: age ≥60, diagnosis of NSCLC confirmed by biopsy, without distant metastases, less than three unilateral lung lesions, single lesion with a diameter less than 5 cm, Eastern Cooperative Oncology Group (ECOG) ≤2, expected survival of over 3 months. The exclusion criteria were determined as follows: age <60, cachexia, systemic metastases, coagulation dysfunction, platelet count <20.0×109/L, unable to tolerate percutaneous lung puncture, severe cardiopulmonary dysfunction, and severe cognitive impairment or other factors which resulted in failure to complete the procedure.
Chemotherapy
In group A, patients with squamous cell carcinomas received single-agent docetaxel (75 mg/m2/3 weeks) or gemcitabine (1000 mg/m2 on days 1 and 8), and with adenocarcinoma received single-agent pemetrexed (500 mg/m2/3 weeks). In group B, patients with squamous cell carcinomas received platinum-based combination chemotherapy, using 75 mg/m2 of docetaxel on day 1, or 1000 mg/m2 of gemcitabine on days 1 and 8, followed by 30 mg/m2 of cisplatin on days 1 to 3 or carboplatin at a dose of 300 to 350 mg/m2 administered on day 2. While patients with adenocarcinoma received 500 mg/m2 of pemetrexed on day 1, combined with carboplatin at a dose of 300–350 mg/m2 administered on day 2. The regimen had a 21-day schedule which was repeated for six cycles, if tolerated. In group A, 125I seed implantation was performed during the interval between chemotherapy.
125I Seeds Implantation Brachytherapy Instruments and Equipment
The 125I sealed seed source was provided by Tianjin Saide Biological Pharmaceutical Co., Ltd, China. The Ag rod with 125I was mounted in the titanium tube and sealed at both ends. The length of the rod was 4.50 mm and the external diameter was 0.80 mm. The matched peripheral dose was 110 to 140 Gy, and the average energy was 27 to 32 KeV. With an initial activity of 0.8 mCi, a radius of 1.7 cm and a half-life of 59.6 days, when an 125I seed was implanted in the lesion, it released continuous low-dose γ-ray and soft X-ray, and 93% to 97% of brachytherapy dose was delivered in 8–10 months. The radioactive particle implantation treatment system was provided by Zhuhai Hokai Medical Instruments Co., Ltd, China. The 18G puncture needles were from Cook Medical, Bloomington, IN, USA. The 64-slice CT scanner was from Siemens, Germany.
125I Seeds Implantation Process
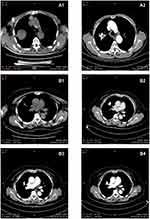
Before 125I seed implantation, an enhanced CT scan, 5-mm thick, was necessary in all 125I seed implantation patients. The CT data were inputted to a treatment planning system (TPS) (Zhuhai Hokai Medical Instruments Co., Ltd, China) to delineate the target region, determine the dose of radioactive seeds implanted, calculate the number of puncture needles, plan the puncture path, measure the needle insertion depth, and calculate the total number of 125I seeds that needed to be implanted. On the CT image, the radiotherapy physician delineated the gross tumor volume (GTV), planned target volume (PTV), and defined surrounding vital organs (spinal cord, thoracic aorta, pulmonary artery, bronchus, etc.). A 5 mm margin was added to the GTV to create PTV, as has been recommended in earlier studies.23 But PTV was defined as a 1.5 cm external expansion to the GTV now.24 The prescribed dose was an averaged 120 Gy (100–140 Gy). Based on the three orthogonal diameters within the target tumor and a prescribed tumor matched peripheral dose (MPD) of average 120 Gy, TPS generated a dose-volume histogram, isodose curves of different percentages, and calculated the dose and number of implanted 125I seeds. PTV edge was covered by an isodose curve from 70% to 90%. The MPD on surrounding vital organs and tissues was reduced according to the following criteria: large vessels 80 Gy, heart 45–50 Gy, oesophagus 60 Gy, trachea 50 Gy, spinal cord 45–50 Gy, breast 50 Gy, thyroid 45 Gy, kidney 20 Gy, and skin 50 Gy.25
After the sterilization, radiation leak, and activity detection, the 125I seeds and disposable implant gun were sent to the operating room, carefully operated to prevent radioactive contamination. Before the operation, a CT scan, 5-mm thick, was obtained to ensure the location and upper and lower borders of the tumor, according to the TPS preoperative plan. The puncture position of the patient’s body surface was marked, and the patient was secured, with care to avoid the ribs, major blood vessels, bronchi, and spinal cord, according to the preoperative CT examination results. The range of needle arrangement and the number of puncture points were determined by combining CT scan positioning lines and body surface markers. The spacing between puncture points was set to about 1 cm. After local anesthesia with 2% lidocaine, an 18-G spinal needle was inserted to reach the farthest tumor edge, but was kept at approximately or less than 5 mm of the border. Similarly, other needles of the same size were punctured into the tumor, with the distance between each needle being approximately 1 cm, checking insertion depth and needle tip position with CT and adjusting as required. From deep to shallow, the 125I seeds were released while retracting back the needle and keeping adjacent particles at a distance of approximately 1 cm. To avoid complications during the operation, all spinal needles were retained until the completion of the implantation and removed at the same time. After implantation of the 125I seeds, the CT scan was performed to assess postoperative complications, such as bleeding and pneumothorax. The last CT scan images were also imported in the TPS program to verify the location of the 125I seed implantation, and evaluate the dose distribution to the tumor area and the surrounding normal tissue. The aim was to ensure that the total activity of the implanted 125I seeds met the prescribed dose of the treatment plan. If the dose requirements were not met, reimplantation was conducted.
Evaluation Criteria
Patients who accepted 125I seed implantation underwent electrocardio monitoring in the radiation interventional ward for 1–2 days. All patients underwent a follow-up examination except for those who died or were lost to follow-up. Physical, laboratory, and imaging examinations of the patients were recorded in detail. Enhanced CT scans, that were performed to evaluate the therapeutic effectiveness, were performed every month for the first 3 months, then once every 3 months. The effectiveness was evaluated according to the response evaluation criteria in solid tumors (RECIST).26 Complete response (CR) was defined as the complete disappearance of all target lesions. A partial response (PR) was defined as at least a 30% decrease in the sum of the diameters of target lesions, taking as reference the baseline sum diameters. Progressive disease (PD) was defined as an increase of at least 20% in the sum of the diameters of target lesions, taking as reference the smallest sum on study. In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm. Stable disease (SD) was defined as neither a sufficient shrinkage to qualify for PR nor a sufficient increase to qualify for PD, taking as a reference the smallest sum diameters while on study. Total response rate (RR) was the sum of the cases of CR+cases of PR/case number. Long-term effectiveness was evaluated according to 1-year and 2-year survival rates. For calculation of the survival rate, deaths from any cause were scored as events. PFST was defined as the time from the first treatment to the first documented disease progression, death, or end of the study. OS was calculated as the time from acceptance of the first treatment to last follow-up or death of any cause. The toxicity criteria of the World Health Organization (WHO) were applied to assess the chemotherapy-related adverse effects. The acute and late adverse effects of irradiation were assessed according to the Radiation Therapy Oncology Group (RTOG).
Statistical Analysis
The statistical software package SPSS version 23.0 was used for statistical analyses. Data were expressed as means±standard deviation. The significance of differences was analyzed using the t-test. The response to therapy and adverse effect were evaluated using the Wilcoxon test or x2 test. Survival analysis was assessed using the Kaplan–Meier method and the Log rank test. A P-value<0.05 was considered as an indicator of a statistically significant difference.
Results
Response Rate
In group A, 125I seeds implantation and six cycles of single-agent chemotherapy were well tolerated by all patients. But six of 60 patients failed to complete six cycles of combination chemotherapy in group B, because of the toxic side-effects of chemotherapy, which subsequently received only symptomatic and supportive care. After the 6-month follow-up of patients, the CR was 14 (28.0%) vs 0 (0%); PR was 25 (50.0%) vs 28 (46.7%); SD was 8 (16.0%) vs 22 (36.7%); PD was 3 (6.0%) vs 10 (16.6%); and RR was 39 (78.0%) vs 28 (46.7%) in both groups A and B (Figure 1). The total RR in tumor response accounted for 78.0% in group A, which was significantly higher than that of group B (P<0.0001; Table 2). According to the imaging results, when the lesion was still enhanced or active, 125I seed was re-implanted accompanied by intermittent single-agent chemotherapy in group A. In group B, patients with active lesions received chemotherapy again if tolerated.
 |
Table 2 Comparison of Treatment Effect in Groups A and B (Wilcoxon Test) |
PFST and Overall Survival
Follow-up ranged from 3–60 months, the median follow-up time was 19.06±0.76 months (95% confidence interval [CI]=18.32–20.64). The median PFST was 15.08±0.85 months (95% CI=13.42–16.74) in group A and 10.03±0.53 months (95% CI=9.01–11.06) in group B (x2=24.18, P=0.000, Log rank test; Figure 2). The difference between groups A and B was statistically significant.
 |
Figure 2 Kaplan–Meier curve of progression-free survival of patients in both groups A and B. |
The 1- and 2-year OS rates for group A were 88.00% and 54.00%, respectively, and for group B, 71.67%, and 13.33%, respectively. The median OS time was 23.71±1.41 months (95% CI=20.95–26.47) for group A and 16.12±0.93 months (95% CI=14.31–17.93) for group B, respectively (x2=17.68, P=0.000, Log rank test; Figure 3). The Log rank test showed a significant difference in OS between two groups.
 |
Figure 3 Kaplan–Meier curve of overall survival of patients in both groups A and B. |
Relief of Clinical Symptoms
The clinical symptoms of NSCLC usually include cough, chest pain, bloody sputum, and chest tightness. But we found changes in these clinical symptoms after treatment. The symptoms of cough and chest pain in group A showed significant relief compared with those in group B (Table 3).
 |
Table 3 Relief of Clinical Symptoms and Laboratory Indicators Associated with NSCLC in Both Groups (Wilcoxon Test) |
Change of Laboratory Index
Patients with NSCLC are usually accompanied by abnormal laboratory indicators, such as carcinoembryonic antigen, squamous cell carcinoma antigen, D-dimer, fibrinogen, and so on. The higher the tumor load, the more obvious the abnormality. After treatment, we also found significant changes in the laboratory index. Compared with group B, the laboratory index of patients in group A significantly improved after treatment, especially regarding carcinoembryonic antigen/squamous cell carcinoma antigen and D-dimer (Table 3).
Complications and Adverse Reactions
In group A, there were no serious complications related to puncture for 125I seeds implantation during our follow-up period. Subcutaneous hemorrhage was common to punctures from seeds implantation, which was easily controlled. Three patients developed pneumonitis which was relieved after anti-inflammatory treatment. Five patients had cough and hemoptysis which improved after treatment with hemostatic drugs and antibiotics. During the procedure, seven patients developed pneumothorax with the lung compressed by less than 30%, which was improved after bed rest and supplemental oxygen. None of the patients underwent radiation pneumonia and 125I seeds did not migrate to other organs. The 125I seed implantation was successful and safe in all the patients in group A.
In both groups A and B, the severe toxicities of chemotherapy were as follows, respectively: myelosuppression (12.00%, 41.67%); gastrointestinal response (8.00%, 33.33%), fever (10.00%, 11.67%), allergy (12.00%, 13.33%), and alopecia (12.00%, 16.67%). There were statistically significant differences in myelosuppression and gastrointestinal responses between the two groups (Table 4).
 |
Table 4 Adverse Reactions of Chemotherapy Observed in Both Groups Upon Follow-Up (X2 Test) |
Discussion
For patients with NSCLC, the tumoricidal dose needs to reach a certain level of 80–120 Gy to control local tumor progression.27 Although an increased external radiation dose can improve local control, it will inevitably lead to damage of normal tissue, and the lowest effective dose could not be reached, which resulted in recurrence and distant metastasis. Fletcher et al28 showed that radiation therapy with 80–100 Gy or more doses for patients with the early stage of large NSCLC had clinical effificacy, but it caused severe radiation-induced pneumonia. The increasing dose of external irradiation is restricted due to the dose-tolerance limits of the normal lung tissue, but 125I seed brachytherapy can provide sufficient radiation for the target regions, with minimal toxicity to the surrounding tissues, which results in relatively high local control rates.29–32 Experimental research has found that 125I interstitial brachytherapy reduced tumor growth by inhibiting the Warburg effect, which may have resulted from downregulation of mTOR, c-Myc, HIF-1α, and GLUT1 expression, particularly c-Myc and GLUT1, in NSCLC A549 xenografts.33 125I particles also can inhibit the growth of HGC-27 gastric cancer cell transplants and promote the expression of Bax, caspase-3, and caspase-8 mRNA in the tumor tissue.34 These studies had confirmed that 125I seed brachytherapy could inhibit the proliferation and promote the apoptosis of cells.35 So when 125I seeds are implanted in the lesions of NSCLC, persistent low-dose irradiation could affect the different stages of cell cycle, which could continue to kill tumor cells.36,37 The low oxygen dependence of γ-rays can improve the killing effect on tumor cells.38 Meanwhile, the low emission rate of the radiation also gives surrounding normal tissue sufficient time to repair from sublethal damage.27 Additionally, the 125I seed brachytherapy can be followed by continuous internal radiotherapy and continuous irradiation for 8–10 months. The 125I seed implantation can be repeated for local lesions as required.
125I seed has an effective radiation only in 1.7 cm distance, and the decay of radiation dose is inversely proportional to the spare of distance between 125I and the target site.39–41 The internal radiation dose promptly declines to a relatively lower level from the outside of lung tumors, which partially protects the healthy lung tissues from radiation injury.38 Huo et al42 reported that if the prescribed dose of 125I seed exceeded 110.0 Gy, it would be effective for patients with stage III NSCLC. In the present study, we found that the accumulative dose reached 110–140 Gy, and the radiation doses were distributed within 1 cm by the TPS, which caused less damage to the normal lung tissue. Local control rate reached 100%, and the duration of local control was >6 months. Another study showed that using a low dosage (7.7 cGy/h) and prolonged radiation time (3–4 half-life periods) of radioactive 125I seed were able to effectively kill tumor cells independent of cell cycle with a minor injury to normal tissue adjacent to the massive lung cancer lesion.39,43
TPS plan and CT scan were key to ensuring the effect and reducing the related complications for NSCLC. Without damaging the surrounding organs, a TPS plan could ensure that 95% of the tumor volume accepted 100% of the prescribed dose (PD), which was consistent with the American Brachytherapy Society’s so-called dual 90 (90% of the tumor volume acquire 90% prescription dose).35,44 CT scan could clearly show tumor location, boundary, important blood vessels and organs, which was helpful to control and reduce complications related to 125I implantation. Now application of 3-D printing template-assisted radioactive 125I seed implantation can help shorten operation time and optimize radiation-dose distribution, which is worthy of promoting. In the present study, there was no case of related death due to 125I implantation.
125I seed brachytherapy has very good effect on tumor local control, but it still has shortcomings. First, different pathological types of tumors have different sensitivity to 125I seed brachytherapy. Second, because of obstacles such as the ribs, scapula, adjacent great vessels and so on, some places in the lung could not be reached completely, so the 125I seed implantation might be difficult for some locations.22 Third, in patients with poor lung function, lung puncture is prone to serious complications. So even with complete preoperative TPS or 3-D printing template, the distribution of 125I seed cannot be guaranteed to be absolutely uniform. There are still tumor remnants after the implantation of 125I seed. Meanwhile, 125I seeds have no effect on distant metastases (such as lymph nodes) from lung cancer. All of these require adjuvant systemic chemotherapy. Chemotherapy as a systemic treatment has the potential role to control tumor, increase operability, and eliminate micrometastases. But it is unsatisfactory that chemotherapy has achieved very small survival benefits and is limited by significant toxicity such as myelosuppression, nausea and vomiting, liver and kidney function damage, cardiotoxicity, skin pigmentation, and hair loss.45,46
If the 125I seed brachytherapy and chemotherapy combined use, is it possible to benefit from each other? 125I seed brachytherapy which control local tumors, can reduce the dose of chemotherapy drugs and the toxic side-effects of chemotherapy. Chemotherapy can control whole body tumor and reduce the tumor size, which is beneficial to 125I seed implantation and cost saving. Yu et al47 reported that combination of 125I brachytherapy and chemotherapy is an efficacious and safe therapy for NSCLC.
As the population ages, elderly lung cancer cases show an increasing trend, which is considered as a important factor for treatment selection. Elderly patients with lung cancer usually suffer from a variety of chronic diseases (eg, chronic obstructive emphysema), poor general condition, and loss of organ-function reserve, all of which tend to be less tolerant to conventional chemotherapy than their younger counterparts. Although chemotherapy can prolong OS compared to best supportive care, considering higher toxicity rates, single-agent chemotherapy was recommended especially for elderly patients, combination chemotherapy recommended according to individual conditions.15,48
Wu et al16 explored the effectiveness of 125I brachytherapy combined with chemotherapy vs chemotherapy alone for stage III or IV NSCLC in the elderly (100 patients were equally divided into two groups), and the PFS was 13 months vs 8 months (P<0.05) with a median follow-up time of 20 months, and no severe complications. The findings of our study show that CT-guided 125I seed brachytherapy combined with single-agent chemotherapy was safe and effective for elderly patients with NSCLC. The 125I seed implantation procedure was successful in group A. No severe procedure related major complications, such as massive hemoptysis, fatal pneumothorax, tumor seeding along the needle tracts, radiation pneumonitis, or death were seen in group A. The median PFST (15.08±0.85 months vs 10.03±0.53 months) and OS (23.71±1.41 months vs 16.12±0.93 months) were significantly longer in group A than in group B. In addition, CT-guided 125I seed brachytherapy combined with single-agent chemotherapy offered better local tumor control and improvement in the quality-of-life. The total RR in tumor response accounted for 78.0% in group A, which was significantly higher than that of group B. The symptoms of cough and chest pain in group A showed significant relief compared with those in group B. The side-effects of single-agent chemotherapy were also less severe.
Conclusion
Our study preliminarily demonstrated that CT-guided 125I seed brachytherapy combined with single-agent chemotherapy was effective and safe for elderly patients with NSCLC. It provided good local control and higher survival rates and relieved clinical symptoms without increasing side-effects. But our study was limited by its retrospective design, sample size, and relatively short follow-up period for some patients. Future research needs to further confirm the efficacy and safety of this treatment with pathological changes and functional imaging evaluation in a larger sample size. Prospective studies should also be carried out.
Acknowledgment
First, we thank all participants and patients who participated in this study. Then we thank Professor Wei Xing for providing statistical advice for the data from the inception of the study especially. This work is supported by the institutional review board at our hospitals (The Affiliated Jianhu Hospital of Nantong University).
Disclosure
The authors declare no conflicts of interest in this research.
References
1. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi:10.3322/caac.21349
2. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
3. Majem M, Juan O, Insa A, et al. SEOM clinical guidelines for the treatment of non‑small cell lung cancer (2018). Clin Transl Oncol. 2019;21(1):3–17. doi:10.1007/s12094-018-1978-1
4. Zhong L, Sun SY, Shi JH, et al. Clinical analysis on 113 patients with lung cancer treated by percutaneous CT-guided microwave ablation. J Thorac Dis. 2017;9(3):590–597. doi:10.21037/jtd.2017.03.14
5. Mouillet G, Monnet E, Milleron B, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol. 2012;7(5):841–849. doi:10.1097/JTO.0b013e31824c7d92
6. Huo XD, Wang HX, Yang JK, et al. Effectiveness and safety of CT-guided (125)I seed brachytherapy for postoperative locoregional recurrence in patients with non-small cell lung cancer. Brachytherapy. 2016;15(3):370–380. doi:10.1016/j.brachy.2016.02.001
7. Lam A, Yoshida EJ, Bui K, et al. Patient and Facility Demographics Related Outcomes in Early-Stage Non-Small Cell Lung Cancer Treated with Radiofrequency Ablation: A National Cancer Database Analysis. J Vasc Interv Radiol. 2018;29(11):1535–1541. doi:10.1016/j.jvir.2018.06.005
8. Iguchi T, Hiraki T, Matsui Y, et al. Survival Outcomes of Treatment with Radiofrequency Ablation, Stereotactic Body Radiotherapy, or Sublobar Resection for Patients with Clinical Stage I Non-Small-Cell Lung Cancer: A Single-Center Evaluation. J Vasc Interv Radiol. 2020;31(7):1044–1051. doi:10.1016/j.jvir.2019.11.035
9. Osmani L, Askin F, Gabrielson E, et al. WHO Guidelines and the Critical Role of Immunohistochemical Markers in the Subclassification of Non Small Cell Lung Carcinoma (NSCLC): moving from Targeted Therapy to Immunotherapy. Semin Cancer Biol. 2018;52:103–109. doi:10.1016/j.semcancer.2017.11.019
10. Gomes JR, Cruz MR. Combination of afatinib with cetuximab in patients with eGFr-mutant non-small-cell lung cancer resistant to eGFr inhibitors. Onco Targets Ther. 2015;8:1137–1142. doi:10.2147/OTT.S75388
11. Liew MS, Sia J, Starmans MH, et al. Comparison of toxicity and outcomes of concurrent radiotherapy with carboplatin/paclitaxel or cisplatin/etoposide in stage III non-small cell lung cancer. Cancer Med. 2013;2(6):916–924.
12. Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–2190. doi:10.1200/JCO.2009.26.2543
13. Cardenal F, Nadal E, Jove M, et al. Concurrent systemic therapy with radiotherapy for the treatment of poor-risk patients with unresectable stage III non-small-cell lung cancer: a review of the literature. Ann Oncol. 2015;26(2):278–288.
14. Buzaglo J, Gayer C, Mallick R, et al. Understanding the experience of living with non-small-cell lung cancer (NSCLC): a qualitative study. J Community Support Oncol. 2014;12(1):6–12. doi:10.12788/jcso.0010
15. Zhang QQ, Wang ZH, Guo J, et al. Comparison of single-agent chemotherapy and targeted therapy to first-line treatment in patients aged 80 years and older with advanced non-small-cell lung cancer. Onco Targets Ther. 2015;8:893–898.
16. Wu CR, Li B, Sun GY, et al. Efficacy and safety of iodine-125 brachytherapy combined with chemotherapy in the treatment of advanced NSCLC in the elderly. Onco Targets Ther. 2018;11:6617–6624. doi:10.2147/OTT.S174457
17. Du E, Wang L, Li CY, et al. Analysis of immune status after iodine-125 permanent brachytherapy in prostate cancer. Onco Targets Ther. 2017;10:2561–2567. doi:10.2147/OTT.S137491
18. Shen XY, Li Y, Zhang YF, et al. An analysis of brachytherapy with computed tomography-guided permanent implantation of iodine-125 seeds for recurrent nonkeratin nasopharyngeal carcinoma. Onco Targets Ther. 2015;8:991–997.
19. Tong LN, Liu P, Huo B, et al. CT-guided 125I interstitial brachytherapy for pelvic recurrent cervical carcinoma after radiotherapy</sup>. Onco Targets Ther. 2017;10:4081–4088. doi:10.2147/OTT.S139571
20. Ma XD, Yang ZY, Jiang S, et al. Effectiveness and safety of a robot-assisted 3D personalized template in 125I seed brachytherapy of thoracoabdominal tumors. J Contemp Brachytherapy. 2018;10(4):368–379. doi:10.5114/jcb.2018.77957
21. Ghassemi F, Sheibani S, Arjmand M, et al. Comparison of Iodide-125 and Ruthenium-106 Brachytherapy in the Treatment of Choroidal Melanomas. Clinical Ophthalmology. 2020;14:339–346. doi:10.2147/OPTH.S235265
22. Zhang SC, Zheng YH, Yu PP, et al. The combined treatment of CT-guided percutaneous 125I seed implantation and chemotherapy for non-small-cell lung cancer. J Cancer Res Clin Oncol. 2011;137(12):1813–1822. doi:10.1007/s00432-011-1048-3
23. Li J, Zhang LJ, Xu WH, et al. Computed tomography-guided implantation of 125I seeds brachytherapy for recurrent multiple pulmonary oligometastases: initial experience and results. J Contemp Brachytherapy. 2017;9(2):132–138. doi:10.5114/jcb.2017.67023
24. Chen YX, Gao F, Hen L, et al. Effectiveness and safety of CT-guided 125I brachytherapy for lung metastasis from hepatocellular carcinoma. Open J Radiol. 2013;3(3):159–164. doi:10.4236/ojrad.2013.33026
25. Xiang ZW, Wang LF, Yan HZ, et al. 125I seed brachytherapy versus external beam radiation therapy for the palliation of painful bone metastases of lung cancer after one cycle of chemotherapy progression. Onco Targets Ther. 2018;11:5183–5193. doi:10.2147/OTT.S154973
26. Lencioni R, Llovet JM. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Sem Liv Dis. 2010;30(1):52–60.
27. Song JJ, Fan XX, Zhao ZW, et al. 125I brachytherapy of locally advanced nonsmall-cell lung cancer after one cycle of firstline chemotherapy: a comparison with best supportive care. Onco Targets Ther. 2017;10:1345–1352. doi:10.2147/OTT.S129903
28. Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a Phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–4839.
29. Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial Phase 3 study. Lancet Oncol. 2015;16(2):187–199.
30. Li W, Dan G, Jiang JQ, et al. Repeated iodine-125 seed implantations combined with external beam radiotherapy for the treatment of locally recurrent or metastatic stage III/IV non-small cell lung cancer: A retrospective study. Radiat Oncol. 2016;11(1):119.
31. Li W, Zheng YF, Li YM, et al. Effectiveness of 125I seed implantation in the treatment of non-small cell lung cancer during R2 resection. Oncol Lett. 2017;14(6):6690–6700.
32. Monk BJ, Tewari KS, Puthawala AA, et al. Treatment of recurrent gynecologic malignancies with iodine-125 permanent interstitial irradiation. Int J Radiat Oncol Biol Phys. 2002;52(3):806–815.
33. Zhang J, Zhu YJ, Dong MJ, et al. Iodine-125 interstitial brachytherapy reduces tumor growth via Warburg effect inhibition in non-small cell lung cancer A549 xenografts. Oncol Lett. 2018;16:5969–5977.
34. Yang Y, Chen AF, Ma J, et al. Effects of radioactive 125I on apoptosis of HGC-27 gastric cancer cells. Oncol Lett. 2019;18(5):4916–4922.
35. Yang G, Peng S, Zhang YL, et al. Cell-based assay system to estimate the effect of 125I seeds on cancer cells: effect of osteopontin. Recent Pat Anticancer Drug Discov. 2014;9(2):258–263.
36. Xiang ZW, Bai MJ, Li GH, et al. Safety and efcacy of 125I brachytherapy for bilateral lung recurrences from hepatocellular carcinoma after resection or ablation. J Cancer Res Clin Oncol. 2019;145:1907–1916.
37. Qu A, Wang H, Li JN, et al. Biological effects of (125)I seeds radiation on A549 lung cancer cells: G2/M arrest and enhanced cell death. Cancer Invest. 2014;32(6):209–217.
38. Zhou DH, Shao LJ, Spitz DR. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1–67.
39. Li W, Guan J, Yang L, et al. Iodine-125 brachytherapy improved overall survival of patients with inoperable stage III/IV non-small cell lung cancer versus the conventional radiotherapy. Med Oncol. 2015;32(1):395.
40. Schwarz SB, Thon N, Nikolajek K, et al. Iodine-125 brachytherapy for brain tumours-a review. Radiat Oncol. 2012;7:30.
41. Reveiz L, Rueda JR, Cardona AF. Palliative endobronchial brachytherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2012;12:CD004284.
42. Huo XD, Huo B, Wang HX, et al. Implantation of computed tomography-guided Iodine-125 seeds in combination with chemotherapy for the treatment of stage III non-small cell lung cancer.J. Contemp Brachytherapy. 2017;9(6):527–534.
43. Odell DD, Kent MS, Fernando HC. Sublobar resection with brachytherapy mesh for stage I non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2010;22(1):32–37.
44. Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1–11.
45. He X, Wang J, Li YM. Efficacy and safety of docetaxel for advanced non-small-cell lung cancer: a meta-analysis of Phase randomized controlled trials. Onco Targets Ther. 2015;8:2023–2031.
46. Ballas MS, Chachoua A. Rationale for targeting veGF, FGF, and PDGF for the treatment of NSCLC. Onco Targets Ther. 2011;4:43–58.
47. Yu XJ, Li J, Zhong XM, et al. Combination of Iodine-125 brachytherapy and chemotherapy for locally recurrent stage III non-small cell lung cancer after concurrent chemoradiotherapy. BMC Cancer. 2015;15:656.
48. Chen JQ, Chen JB, Wu XA, et al. Efficacy of targeted agents in the treatment of elderly patients with advanced non-small-cell lung cancer: a systematic review and meta-analysis. Onco Targets Ther. 2016;9:4797–4803.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.