Back to Journals » OncoTargets and Therapy » Volume 10
125I brachytherapy of locally advanced non-small-cell lung cancer after one cycle of first-line chemotherapy:a comparison with best supportive care
Authors Song J, Fan X , Zhao Z, Chen M, Chen W, Wu F, Zhang D, Chen L, Tu J, Ji J
Received 10 December 2016
Accepted for publication 9 February 2017
Published 2 March 2017 Volume 2017:10 Pages 1345—1352
DOI https://doi.org/10.2147/OTT.S129903
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Jingjing Song* Xiaoxi Fan* Zhongwei Zhao* Minjiang Chen* Weiqian Chen, Fazong Wu, Dengke Zhang, Li Chen, Jianfei Tu, Jiansong Ji
Department of Interventional Radiology, Zhejiang University Lishui Hospital, The Fifth Affiliated Hospital of Wenzhou Medical University, Lishui Central Hospital, Lishui, Zhejiang, People’s Republic of China
*These authors have contributed equally to this work
Objectives: The objective of this study was to assess the efficacy of computed tomography (CT)-guided 125I brachytherapy alone in improving the survival and quality of life of patients with unresectable locally advanced non-small-cell lung cancer (NSCLC) after one cycle of first-line chemotherapy.
Patients and methods: Sixteen patients with locally advanced NSCLC were treated with CT-guided 125I brachytherapy after one cycle of first-line chemotherapy (group A). Sixteen patients who received only best supportive care (group B) were matched up with the patients in group A. Primary end point included survival, and secondary end point included assessment of safety, effectiveness of CT-guided 125I brachytherapy, and improvement in the quality of life.
Results: The two groups were well balanced in terms of age, disease histology, tumor stage, tumor location, and performance status (P>0.05). The median follow-up time was 16 months (range, 3–30). The total tumor response rate was 75.0% in group A, which was significantly higher than that in group B (0.0%) (P<0.01). The median progression-free survival time was 4.80 months for patients in group A and 1.35 months for patients in group B (P<0.001). Kaplan–Meier survival analysis showed that the median survival time of group A was 9.4±0.3 months versus 8.4±0.1 months in group B (P=0.013). Tumor-related symptoms of patients were significantly relieved, and the quality of life was markedly improved in group A than in group B.
Conclusion: CT-guided 125I brachytherapy improved the survival of patients with locally advanced NSCLC and quality of life after one cycle of first-line chemotherapy compared with best supportive care.
Keywords: non-small-cell lung cancer, CT-guided intervention, 125I seed, brachytherapy
Introduction
Lung cancer remains the leading cause of cancer-related mortalities,1,2 among which 85% of deaths are caused by non-small-cell lung cancer (NSCLC).3,4 Up to 70% of NSCLC patients were diagnosed at an advanced stage with no chance of curative resection.5 The expected survival time of patients with locally advanced NSCLC is less than 6 months if patients did not receive any medical intervention.6,7 For patients with stage III NSCLC, treatments are delivered to the patients with a goal of achieving a local tumor control and preventing the systemic metastases.8 Chemotherapy, particularly combined with external beam radiotherapy, can offer some benefits of prolonging overall survival (OS) to the patients with advanced NSCLC.9–11 However, a large number of patients at an advanced tumor stage are not eligible for the currently available treatment modalities due to their poor general conditions and intolerability to severe toxicities after one or more cycles of first-line chemotherapy, especially the severe adverse events occurring in vital organs.12,13 Despite the wide application of sophisticated radiotherapy technologies, such as three-dimensional computerized planning systems, multileaf beam collimators, and altered fractionation schedules, the adverse effects of radiotherapy remain inevitable.14 In the meanwhile, clinical trials have shown that second-line chemotherapy has only limited therapeutic effect on patients with good or intermediate performance status (PS) (0–2).15 Therefore, there is a pressing demand of using effective and less toxic alternative treatments for the patients in despair after the failed chemoradiation therapy.
125I brachytherapy performed by implantation of 125I radioactive seeds in the tumors can induce extensive necrosis of tumors and improve the quality of life of patients.16,17 Previous studies have shown that 125I seed brachytherapy is accepted as a minimally invasive and effective therapy for various tumors,18 such as prostate cancer, pancreatic cancer, liver cancer, gynecologic malignancies, and brain cancers.12,19–21 Recently, a few studies have also attempted to use 125I brachytherapy for managing unresectable locally advanced lung cancers.22 However, most of these studies focused on evaluating the effectiveness of the combination therapy of 125I brachytherapy with chemotherapy or radiotherapy.23,24 In clinical practice, a considerable number of patients decline to receive any antitumor treatments due to various reasons, such as the intolerable severe side effects of chemotherapy or radiation therapy, the poor general conditions caused by multiple comorbidities, and the unaffordable high cost of chemotherapeutic regime. Computed tomography (CT)-guided 125I brachytherapy is accepted as a minimally invasive, relatively cost-effective treatment option for managing unresectable cancers. Currently, no studies have ever investigated the effectiveness of CT-guided 125I brachytherapy alone with regard to improving the OS and quality of life of patients with unresectable locally advanced NSCLC after one cycle of first-line chemotherapy.
The purpose of this study was to assess whether CT-guided 125I brachytherapy alone could result in longer survival than best supportive care for patients with unresectable locally advanced NSCLC after one cycle of first-line chemotherapy. In the meanwhile, the safety and effectiveness of CT-guided 125I brachytherapy in improving the quality of life of patients were investigated as well.
Patients and methods
Study design
This study was reviewed and approved by ethics committee of the Fifth Affiliated Hospital of Wenzhou Medical University according to the standards of the Declaration of Helsinki. The ethics committee of the Fifth Affiliated Hospital of Wenzhou Medical University deemed patient consent not necessary due to the retrospective nature of this study. Written informed consent was obtained for publication of the associated images. All the records of patients were anonymized and deidentified prior to analysis.
Between January 2010 and December 2015, the medical records of 378 NSCLC patients who received comprehensive treatments were reviewed. Sixteen consecutive patients diagnosed with unresectable locally advanced NSCLC were treated with CT-guided 125I brachytherapy (group A) after one cycle of first-line chemotherapy.
Study inclusion criteria were as follows: 1) histologically or cytologically proven unresectable locally advanced NSCLC, 2) unilateral lung lesion with a diameter less than 5 cm, 3) NSCLC in stage III according to the International Union Against Cancer staging system with a PS of 0–2, 4) progressive disease (PD) after one cycle of first-line chemotherapy, 5) life expectancy longer than 3 months, and 6) platelet count >20.0×109/L and normal coagulation function. Exclusion criteria were 1) unable to tolerate percutaneous lung biopsy procedure, 2) severe cardiopulmonary dysfunction, 3) refusal of 125I seed implantation treatment, and 4) treated with any other antitumor treatment (immunotherapy, biologic systemic anticancer therapy, etc.).
A cohort of 69 unresectable locally advanced NSCLC patients who received best supportive care after one cycle of first-line chemotherapy was used as the candidates of subjects in the control group (group B). To obtain a comparable outcome, we selected a subgroup of 16 patients from the 69 patients who matched up very well with the patients in group A in the context of their age, tumor histology characteristics, union for international cancer control tumor node metastasis (UICC TNM) stage, tumor location, and PS.
All the patients received one initial cycle of chemotherapy. The commonly used regimens and dosage were as follows: 1,000 mg/m2 of paclitaxel on days 1, 8, and 15 or 1,000 mg/m2 of gemcitabine on days 1 and 8, followed by 30 mg/m2 of cisplatin on days 1–3.
CT-guided 125I seeds implantation
125I seeds (0.8×4.5 mm2; Seeds Biological Pharmacy Ltd., Tianjin, China) were sealed in 0.05-mm-thick titanium capsules. The core of the radioactive source was a 125I radionuclide silver rod with a diameter of 0.5 mm and a length of 3.0 mm. The 125I seed continuously emits low-dose gamma rays (average energy of 27.4–35.4 keV) with a half-life of 59.6 days, and the matched peripheral dose was 120–140 Gy. The 125I seeds had antineoplastic activity in a radius of 1.7 cm. Within 8–10 months, 93%–97% of the brachytherapy dose was delivered to the tumors.
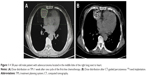
All the patients underwent an enhanced CT scan at a slice thickness of 5 mm 1 week before the 125I seed implantation procedure. These imaging data were integrated into a treatment planning system (TPS; RT-RSI; Beijing Atom and High Technique Industries Inc., Beijing, China) to optimize the dosing scheme of radioactive seeds implanted in the tumor. The radiation therapist formulated the gross tumor volume, planning target volume (PTV, a perimeter including a 0.5–1 cm range surrounding tumor lesions), and surrounding vital organs and vascular location and calculated the minimal prescribed dose (MPD) tumor value, which averaged 120 Gy (100–140 Gy). According to the preoperative plan, we determined the required number and location of the seeds and marked the puncture access of the needle on the patient’s body surface (Figure 1). Preprocedure breath training was performed to ensure a smooth and steady breath movement during the procedure. Then, we chose the shortest puncture pathway to minimize the incidence of pneumothorax, as well as avoiding puncture of critical organs. Under CT-imaging guidance, an 18-gauge needle was gradually inserted into the tumor, and the turntable implantation gun was used to implant seeds into the tumor at 0.5–1.0 cm intervals. CT scan postseed implantation was performed to examine any postoperative complications, such as pneumothorax and hemothorax. The imaging data were exported into the TPS program to verify the location of particles and sufficient MPD. Patients who accepted 125I seed implantation underwent continuous electrocardiograph monitoring for a duration of 24 h. All postoperative symptoms were recorded, and patients received the necessary medication for managing the uncritical symptoms.
BSC treatment
Patients in group B only received palliative supportive treatments after one cycle of first-line chemotherapy including administration of antibiotics and analgesic drugs of transfusions.
Imaging follow-up
Enhanced CT scan was performed once per month for the first 3 months and then once every 3 months after the procedure. Follow-up imaging examinations were performed to evaluate the therapeutic effectiveness of 125I brachytherapy. The treatment outcome of all patients was evaluated, including the overall response rate, progression-free survival time (PFST), OS, and treatment-related complications.
Evaluation criteria
The effectiveness of 125I brachytherapy was evaluated according to the response evaluation criteria in solid tumors.25 The complete response (CR), partial response (PR), stable disease (SD), and PD were classified accordingly. The overall response rate was calculated using the following formula: response rate = the sum of CR cases plus PR cases/the total patient number. PFST was defined as the time interval from the time point of the initiation of the treatment to the time point of documented disease progression, death, or end of the study. OS was defined as the time from the first treatment to death. Adverse effects of irradiation were evaluated according to the Toxicity Criteria of the Radiation Therapy Oncology Group.
Study end points
The primary end point of the study was survival, and the secondary end point included assessment of safety, effectiveness (CT-guided 125I seed implantation), and patients’ quality of life.
Statistical analysis
All statistical analyses were performed using the SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). Continuous data were expressed as mean ± SD. Continuous variables of the two groups were compared using Student’s t-test. Categorical variables were analyzed using the χ2 test or Fisher’s exact test. Survival of patients in two groups was analyzed using the method of Kaplan–Meier survival analysis and compared using the log-rank test. All reported P-values are two sided. A P-value <0.05 was considered as statistically significant.
Results
Baseline characteristics and technique effectiveness
Of the 32 patients, 23 (71.9%) were men and 9 (28.1%) were women. The two groups were well balanced in terms of age, tumor classification, tumor stage, and patient PS. The demographic and disease characteristics of patients are shown in Table 1. The procedures of 125I seed implantation were performed successfully in all patients in group A, and the average number of seeds implanted per patient was 18 (range: 6–43).
Response rate and survival outcomes
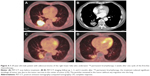
The median follow-up time was 16 months (range: 3–30). The median tumor diameter of the patients in group A was 4.1 cm (range: 3.6–4.6 cm) and 3.2 cm in group B (range: 2.4–3.8 cm) (t=4.370, P=0.242). The implanted 125I seeds yielded a mean D90 of 109 Gy (range: 81–138 Gy) according to the posttreatment dosimetric measurement. After 6-month follow-up of the patients in both groups, CR and PR were observed in 7/16 (43.8%) and 5/16 (31.3%) patients in group A and 0/16 (0.0%) and 0/16 (0.0%) in group B (χ2=8.96, P=0.003; χ2=5.926, P=0.015), 3/16 patients with PD (18.8%) in group A versus 13/16 patients (81.3%) in group B. The tumors of 12/16 (75.0%) patients in group A had total response to the treatment versus 0/16 (0.0%) in group B (χ2=19.20, P<0.001; Table 2). The median PFST of patients in group A was 4.80 months (95% confidence interval [CI]: 4.61–4.99) and 1.35 months (95% CI: 1.01–1.59) in group B (log-rank test, χ2=36.10, P<0.001; Figure 2). The median OS time was 9.4±0.30 (95% CI: 8.81–9.99) months in group A and 8.4±0.10 (95% CI: 8.21–8.60) months in group B (log-rank test, χ2=6.114, P=0.013; Figure 3).
Complications
The needle puncture procedure caused pneumothorax with pulmonary collapse of less than 30% of the unilateral lung volume in two patients in group A, who did not need tube drainage. Two patients developed minor bleeding at the needle puncture site, which was controlled by pressing the puncture site with gauze. No incidences of seed migration and substantial radiation pneumonitis were seen in the patients of group A. The 125I seeds were implanted successfully into the tumors of all the patients in group A. No procedure-related deaths occurred in the patients of group A.
Relief of clinical symptoms
The frequently seen symptoms of patients with stage III NSCLC include cough, chest pain, bloody sputum, hoarseness, and chest tightness. The symptoms of patients in group A significantly improved compared with the patients in group B (Table 3, P<0.01). In group A, we found obvious relief of cough in seven patients, chest pain relief in five patients, moderate cough relief in four patients, and moderate chest pain relief in two patients. In group B, no patients had significant symptom improvement. Improvement in the symptoms of bloody sputum, hoarseness, and chest tightness was seen in 9/12, 6/8, and 8/11 patients in group A, respectively, and no patients had the symptom improvement in group B.
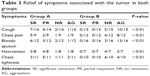  | Table 3 Relief of symptoms associated with the tumor in both groups |
Discussion
The findings of our study show that CT-guided 125I brachytherapy is a safe and effective treatment for patients with unresectable locally advanced NSCLC after one cycle of first-line chemotherapy. The 125I seed implantation procedure was successful in all of the 16 patients. No severe procedure-related major complications, such as massive hemoptysis, tumor seeding along the needle tracts, radiation pneumonitis, or death were seen in the patients. The median PFST and OS time were significantly longer for the patients with 125I seed brachytherapy than best supportive care alone group. In addition, the treatment of intratumoral 125I seed brachytherapy offered better local tumor control and improvement in the quality of life (Figure 4).
For patients with locally advanced NSCLC, the tumoricidal dose needs to reach a certain level of 80–120 Gy to control tumor progression.26 Although the increasing radiation dose of external radiation could significantly improve the local control rate, it will inevitably lead to damage of normal tissue simultaneously, and the lowest effective dose could not be reached.27 These factors resulted in locally advanced NSCLC recurrence and distant metastasis.
As an internal radiotherapy, 125I permanent brachytherapy has been reported since 1940s, which has been successfully used in the treatment of prostate cancer, glioma, pancreatic cancer, hepatocellular carcinoma, and head and neck neoplasms.19–21,28,29 Recently, CT-guided 125I seed implantation has been attempted to treat NSCLC.30 Zhang et al31 found that CT-guided 125I radioactive seed implantation combined with chemotherapy of gemcitabine plus cisplatin are effective and safe for treating advanced NCSLC. The 1- and 2-year survival rates were 62.5% and 16.7% in the combination therapy group and 41.4% and 13.8% in the chemotherapy alone group, respectively. Jiang et al32 reported that interstitial permanent brachytherapy of 125I seeds under CT guidance is a feasible, effective, and safe modality for patients with recurrence of NSCLC after external beam radiation therapy. The estimated median OS is 18 months (95% CI: 11.91–24.09 months). The 1- and 2-year OS rates were 67.5% and 27.0%, respectively. A few studies showed that 125I permanent brachytherapy has been accepted as a safe and effective treatment in patients with unresectable NSCLC, offering a long-term benefit of local tumor control and improvement in the quality of life.33,34 However, these studies involved the combination therapy of 125I permanent brachytherapy with chemotherapy or external beam radiation therapy. The benefits may attribute to the mixed therapeutic effect of both therapies. No studies have investigated the efficacy of 125I permanent brachytherapy alone in prolonging OS and improving the quality of life of the patients with NSCLC. Our study retrospectively compared the outcome of the intention-to-treatment patients receiving 125I permanent brachytherapy alone with those patients who declined to receive further antitumor treatment after one cycle of chemotherapy other than best supportive care. Our study preliminarily indicates that 125I permanent brachytherapy can contribute alone to a prolonged OS time, PFST, and improved quality of life.
125I permanent brachytherapy has marked advantages compared with external beam radiation therapy. The intratumorally implanted 125I seeds can locally deliver high dose of gamma rays to tumor cells with minimal irradiation to adjacent critical structures.35 Permanent low-dose irradiation can continuously exert the antineoplastic effect on the tumor cells in different cell cycles.36 Meanwhile, the low oxygen dependence of gamma rays can improve the killing effect on tumor cells.37 Additionally, the low emission rate of the radiation also gives surrounding normal tissue sufficient time to repair from sublethal damage, protecting healthy organs from late tissue damage.38,39
In our study, the median PFST and survival time were much higher in group A than group B, with significant difference revealed by Kaplan–Meier analysis. There may be two explanations for this result. First, 125I seed implantation with the preoperative TPS allows the PTV to cover >95% of the tumor volume, which will receive 100% of the radiation dose. Therefore, we could guarantee the administration of a sufficiently high dose to the local tumor without damaging the surrounding tissue. Second, the relatively long half-life (59.6 days for 125I seeds) provides prolonged radiation exposure to the seed-implanted tumor volume, with a longer treatment time.
Very few 125I seed implantation-caused complications occurred in the patients treated by 125I seed brachytherapy. This may attribute to several factors: 1) preprocedure breath training was essential to ensure a smooth and steady breath movement during the procedure. 2) We chose the shortest possible needle trajectory in healthy lung tissue. 3) Enhanced CT images were very useful as a reference for guiding the needle placement, which could prevent puncturing into the vessels, trachea, and bronchus. 4) We used a single needle to release seeds. After the needle was located in the center of the tumor, we withdrew the needle to the margin of the tumor and then advanced the needle in difference angles into the tumor for releasing seeds. 5) CT scan postseed implantation was performed to ensure there were no postoperative complications, such as pneumothorax and hemothorax.
Our study has a few limitations. This is a retrospective study with a small sample size due to the small number of patients who had the intention to receive the 125I permanent brachytherapy alone and patients who gave up the antitumor treatment because of various reasons. Our study focused on investigating the single efficacy of 125I permanent brachytherapy with respect to the benefits of improving the OS and progression-free survival. We did not compare the outcome of 125I permanent brachytherapy with any other antitumor therapies, such as systemic chemotherapy and chemoradiation therapy. Our study provided a useful clue that it is feasible to launch a well-designed, multicenter, randomized clinical trial involving the comparison of 125I permanent brachytherapy with systemic chemotherapy for patients with unresectable, locally advanced NSCLC.
Conclusion
CT-guided 125I permanent brachytherapy alone can offer some benefits of prolonging survival to patients with unresectable localized advanced NSCLC after one cycle of first-line chemotherapy. For patients who refuse to receive any other antitumor treatments, 125I permanent brachytherapy is an acceptable choice of treatment to achieve a local tumor control with less adverse events.
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. | ||
Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. | ||
Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25(35):5570–5577. | ||
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Paper presented at: Mayo Clinic Proceedings; 2008. | ||
Dang J, Li G, Zang S, Zhang S, Yao L. Comparison of risk and predictors for early radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with radiotherapy with or without surgery. Lung Cancer. 2014;86(3):329–333. | ||
Yamaguchi M, Sugio K. Current status of induction treatment for N2-Stage III non-small cell lung cancer. Gen Thorac Cardiovasc Surg. 2014;62(11):651–659. | ||
Reinmuth N, Payer N, Muley T, et al. Treatment and outcome of patients with metastatic NSCLC: a retrospective institution analysis of 493 patients. Respir Res. 2013;14:139. | ||
Cardenal F, Nadal E, Jove M, Faivre-Finn C. Concurrent systemic therapy with radiotherapy for the treatment of poor-risk patients with unresectable stage III non-small-cell lung cancer: a review of the literature. Ann Oncol. 2015;26(2):278–288. | ||
Liew MS, Sia J, Starmans MH, et al. Comparison of toxicity and outcomes of concurrent radiotherapy with carboplatin/paclitaxel or cisplatin/etoposide in stage III non-small cell lung cancer. Cancer Med. 2013;2(6):916–924. | ||
Wagner TD, Yang GY. The role of chemotherapy and radiation in the treatment of locally advanced non-small cell lung cancer (NSCLC). Curr Drug Targets. 2010;11(1):67–73. | ||
Ebara S, Katayama N, Tanimoto R, et al. Iodine-125 seed implantation (permanent brachytherapy) for clinically localized prostate cancer. Acta Med Okayama. 2008;62(1):9–13. | ||
Cannon DM, Mehta MP, Adkison JB, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2013;31(34):4343–4348. | ||
Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–4839. | ||
Gridelli C, Ardizzoni A, Ciardiello F, et al. Second-line treatment of advanced non-small cell lung cancer. J Thorac Oncol. 2008;3(4):430–440. | ||
Wang KX, Jin ZD, Du YQ, et al. EUS-guided celiac ganglion irradiation with iodine-125 seeds for pain control in pancreatic carcinoma: a prospective pilot study. Gastrointest Endosc. 2012;76(5):945–952. | ||
Guo J-H, Teng G-J, Zhu G-Y, et al. Self-expandable esophageal stent loaded with 125I seeds: initial experience in patients with advanced esophageal cancer 1. Radiology. 2008;247(2):574–581. | ||
Bagshaw MA, Cox RS, Ramback JE. Radiation therapy for localized prostate cancer. Justification by long-term follow-up. Urol Clin North Am. 1990;17(4):787–802. | ||
Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40(04):314–320. | ||
Fu-Jun Z, Chuan-Xing L, Liang Z, Pei-Hong W, De-Chao J, Guang-Feng D. Short-to mid-term evaluation of CT-guided 125I brachytherapy on intra-hepatic recurrent tumors and/or extra-hepatic metastases after liver transplantation for hepatocellular carcinoma. Cancer Biol Ther. 2009;8(7):585–590. | ||
Hu X, Qiu H, Zhang L, et al. Recurrent gliomas: comparison of computed tomography (CT)-guided 125I seed implantation therapy and traditional radiochemotherapy. Cancer Biol Ther. 2012;13(10):840–847. | ||
Martinez-Monge R, Pagola M, Vivas I, Lopez-Picazo JM. CT-guided permanent brachytherapy for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC). Lung Cancer. 2008;61(2):209–213. | ||
Serrano N, Moghanaki D, Asher D, et al. Comparative study of late rectal toxicity in prostate cancer patients treated with low-dose-rate brachytherapy: with or without supplemental external beam radiotherapy. Brachytherapy. 2016;15(4):435–441. | ||
Shi L, Li X, Pei H, et al. Phase II study of computed tomography-guided (125)I-seed implantation plus chemotherapy for locally recurrent rectal cancer. Radiother Oncol. 2016;118(2):375–381. | ||
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. | ||
Ibahim MJ, Crosbie JC, Yang Y, et al. An evaluation of dose equivalence between synchrotron microbeam radiation therapy and conventional broadbeam radiation using clonogenic and cell impedance assays. PLoS One. 2014;9(6):e100547. | ||
Ayadi M, Zahra N, Thariat J, et al. [Intensity-modulated radiation therapy in non-small cell lung cancers]. Cancer Radiother. 2014;18(5–6):406–413. | ||
Yorozu A, Kuroiwa N, Takahashi A, et al. Permanent prostate brachytherapy with or without supplemental external beam radiotherapy as practiced in Japan: outcomes of 1300 patients. Brachytherapy. 2015;14(2):111–117. | ||
Jiang YL, Meng N, Wang JJ, et al. CT-guided iodine-125 seed permanent implantation for recurrent head and neck cancers. Radiat Oncol. 2010;5(1):1. | ||
Wang Z-M, Lu J, Liu T, Chen K-M, Huang G, Liu F-J. CT-guided interstitial brachytherapy of inoperable non-small cell lung cancer. Lung Cancer. 2011;74(2):253–257. | ||
Zhang S, Zheng Y, Yu P, et al. The combined treatment of CT-guided percutaneous 125I seed implantation and chemotherapy for non-small-cell lung cancer. J Cancer Res Clin Oncol. 2011;137(12):1813–1822. | ||
Jiang W, Wang J, Jiang Y, Li J. CT-guided 125I seed implantation for in-field recurrence of NSCLC with prior radiation. Brachytherapy. 2015;14:S102. | ||
Lee W, Daly BD, DiPetrillo TA, et al. Limited resection for non-small cell lung cancer: observed local control with implantation of I-125 brachytherapy seeds. Ann Thorac Surg. 2003;75(1):237–242; discussion 242–243. | ||
Xiang Z, Li G, Liu Z, et al. 125I brachytherapy in locally advanced nonsmall cell lung cancer after progression of concurrent radiochemotherapy. Medicine (Baltimore). 2015;94(49):e2249. | ||
Monk BJ, Tewari KS, Puthawala AA, Syed AN, Haugen JA, Burger RA. Treatment of recurrent gynecologic malignancies with iodine-125 permanent interstitial irradiation. Int J Radiat Oncol Biol Phys. 2002;52(3):806–815. | ||
Qu A, Wang H, Li J, et al. Biological effects of (125)I seeds radiation on A549 lung cancer cells: G2/M arrest and enhanced cell death. Cancer Invest. 2014;32(6):209–217. | ||
Zhou D, Shao L, Spitz DR. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1–67. | ||
Zheng L, Zhang J, Song T, Zhang J, Yu G, Zhang Y. 125I seed implant brachytherapy for the treatment of parotid gland cancers in children and adolescents. Strahlenther Onkol. 2013;189(5):401–406. | ||
Wallner K, Roy J, Harrison L. Tumor control and morbidity following transperineal iodine 125 implantation for stage T1/T2 prostatic carcinoma. J Clin Oncol. 1996;14(2):449–453. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.