Back to Journals » Cancer Management and Research » Volume 10
12-HETE facilitates cell survival by activating the integrin-linked kinase/NF-κB pathway in ovarian cancer
Authors Liu Q, Tan W, Che J, Yuan D, Zhang L, Sun Y, Yue X, Xiao L, Jin Y
Received 16 July 2018
Accepted for publication 29 September 2018
Published 16 November 2018 Volume 2018:10 Pages 5825—5838
DOI https://doi.org/10.2147/CMAR.S180334
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Qian Liu,1,* Wenhua Tan,1,* Jianhua Che,1,* Dandan Yuan,1 Liying Zhang,1 Yuhong Sun,1 Xiaolong Yue,2 Lei Xiao,3 Yuxia Jin1
1Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang 150001, China; 2Department of Medical Oncology, Affiliated Tumor Hospital of Harbin Medical University, Harbin, Heilongjiang 150001, China; 3Department of General Surgery, The Fourth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang 150001, China
*These authors contributed equally to this work
Background: The dysfunction of cell apoptosis is an important event in the progression of cancer, and the growth of cancer cells is negatively regulated by cell apoptosis. In different types of cancers, inhibition of cellular apoptosis is often observed in the cancerous tissue, and increased resistance to apoptosis is a hallmark of cancer. Although previous studies have shown that 12-lipoxygenase (12-LOX)/12-hydroxyeicosatetraenoic acid (12-HETE) is activated and upregulated in different types of cancers, the consequences of 12-LOX/12-HETE upregulation and its precise roles in the survival of ovarian carcinoma cells are still unknown.
Methods: MTT assays, caspase activity assays, lactate dehydrogenase (LDH) assays, and Western blot analysis were the methods used in this study.
Results: In our study, we found that 12-HETE, a major metabolic product of arachidonic acid using 12-LOX catalysis, inhibited cell apoptosis in a dose-dependent manner and that the effects of 12-HETE on cell apoptosis were mediated by the integrin-linked kinase (ILK) pathway. Moreover, the downstream target of 12-HETE-activated ILK was nuclear factor kappa-B (NF-κB) in ovarian carcinoma. The inhibitory effects of 12-HETE on cell apoptosis were attenuated by the inhibition of the NF-κB pathway.
Conclusion: These results indicate that 12-HETE participates in the inhibition of cell apoptosis by activating the ILK/NF-κB pathway, implying an important underlying mechanism that promotes the survival of ovarian cancer cells.
Keywords: 12-HETE, ILK, apoptosis, NF-κB, ovarian cancer
Background
Ranking fifth among all the causes of cancer-related deaths in women, ovarian cancer is associated with the highest mortality rate among gynecological malignancies.1 The major treatment for ovarian cancer is cytoreductive surgery (debulking) followed by chemotherapy (platinum-based drugs). Unfortunately, symptoms usually do not appear until the disease has spread outside the ovaries, which leads to its late diagnosis and poor prognosis. In addition, a large number of patients with ovarian cancer lose the chance to undergo the operation because of hysteretic diagnosis.2,3 Therefore, targeted drug therapy has become increasingly important in the treatment of ovarian cancer. This situation requires us to conduct more research to define the molecular mechanism regulating the progression of ovarian cancer and to provide novel treatment targets for improving the therapeutic strategy.
Arachidonic acid (AA), a polyunsaturated omega-6 fatty acid, is a component of the phospholipid domain of most cell membranes. Three main pathways, including the cyclooxygenase (COX) pathway, the lipoxygenase (LOX) pathway, and the cytochrome P450 pathway, can metabolize AA to eicosanoids. Humans have three major LOX isoforms: 5-LOX, 12-LOX, and 15-LOX.4,5 The LOX pathways produce several products that exert numerous physiological and pathological effects.6 Among the three LOX isoforms, 12-LOX and its metabolite 12-hydroxyeicosatetraenoic acid (12-HETE) have been reported to advance tumorigenesis and participate in regulating the growth of cancer cells, angiogenesis, interactions between tumor cells and the vasculature, tumor cell mobility, invasion, and proteolysis.7,8 However, the exact role of 12-HETE in the survival of ovarian carcinoma cells and the corresponding molecular mechanism are still poorly defined.
Integrin-linked kinase (ILK) is an intracellular protein serine/threonine kinase that is involved in coordinating the cell signaling pathway regulated by integrins and growth factors, including insulin-like growth factor-I, nerve growth factor, platelet-derived growth factor, and vascular endothelial growth factor.9,10 Moreover, because of its kinase activity, ILK possesses regulatory effects on many physiological functions, such as cell growth and death, cell cycle progression, epithelial-mesenchymal transition (EMT), invasion and migration, cell motility, cell contraction, vascular development, and tumor angiogenesis.11 Previous studies have demonstrated that ILK is closely related to the invasive features, epithelial–mesenchymal transition, and tumorigenesis in ovarian cancer,12–14 but the upstream regulator and downstream target of ILK remain to be explored.
In this study, we found that 12-HETE activated the ILK pathway and prevented mitochondria-dependent apoptosis in ovarian cancer cells. Moreover, the activation and nuclear translocation of nuclear factor kappa-B (NF-κB) facilitated by 12-HETE were mediated by the ILK pathway. In addition, inhibition of the NF-κB pathway could mitigate the inhibitory effects of 12-HETE on cell apoptosis. All our results indicate that 12-LOX/12-HETE and its downstream target pathway (the ILK/NF-κB pathway) are involved in positively regulating the growth and survival of ovarian cancer cells, which implies an important regulatory mechanism affecting ovarian cancer cell growth and provides a potential strategy for the treatment of ovarian cancer.
Materials and methods
Materials
The antibodies against ILK (sc-13075), Bcl-2 (sc-492), Bax (sc-493), beta-actin (sc-1616), GAPDH (sc-25778), NF-κB p65 (sc-372), IKB alpha (IKBa; sc-371), and IKBa (ser32; sc-7977) were purchased from Santa Cruz Biotechnology Inc., CA, USA. The antibodies against Histone 1.2 (ab17677) and NF-κB p65 (phospho-S536; ab86299) were purchased from Abcam (Shanghai, China). Kits for measuring caspase-3 (C1116) and caspase-9 (C1158) activities and a lactate dehydrogenase (LDH) assay kit (C0017) were obtained from Beyotime Institute of Biotechnology (Haimen, China). All other reagents were obtained from common commercial sources.
Cell culture
OVCAR-3 and SKOV3 cells were obtained from ATCC. The cells were maintained in DMEM supplemented with 10% FBS, 1% penicillin, and 1% streptomycin in 5% CO2 atmosphere at 37°C.
siRNA design and transfection
OVCAR-3 and SKOV3 cells were transfected with two ILK small interfering RNAs (siILK and siILK#2), respectively, and a nontargeted control siRNA (siNC) was used as a negative control. Both ILK siRNAs and siNC were designed and synthesized by GenePharma, Shanghai, China. The transfection was conducted as described below. First, OVCAR-3 cells were cultured in 10% FBS DMEM until 30%–50% confluent. After the cells were cultured in serum-free DMEM overnight, 1.5 µg siRNAs and 10 µL X-treme Gene Transfection Reagent were added to serum-free Opti-MEM-1 and gently mixed. Finally, the mixture was incubated at room temperature for 20 minutes and added directly onto cells. After transfection, cells were rested for 24 hours and then used as required.
MTT assay
Cells (OVCAR-3 and SKOV3) were cultured in 96-well plates at a density of approximately 1×104 cells per well. After culturing in 10% FBS DMEM medium for 8–12 hours, the cells were cultured in DMEM without serum overnight and treated with the indicated reagents by group in low-glucose DMEM without FBS (serum deprivation [SD]). Solvent control and other agents were added at the indicated concentrations every 24 hours. After being treated as different groups for 48 hours, the cells were incubated in 0.5% MTT, which is a yellow mitochondrial dye and is dissolved in sterile PBS buffer, for 4 hours at 37°C, and then, the reaction was terminated by incubating the cells with DMSO for 10 minutes. The spectrophotometer absorbance at 540 nm was measured. The amount of blue formazan dye formed from MTT is proportional to the number of surviving cells.
LDH assay
The activity of LDH was used to detect the levels of LDH, which was released into the culture media, and was measured by a cytotoxicity detection kit from Beyotime Institute of Biotechnology. The proportion of injured cells in the cultures was determined by comparing the LDH activity of the medium with the LDH activity after complete cell lysis or total LDH activity. The maximum LDH activity was determined using medium containing Triton-lysed cell supernatants. The experiments were carried out according to the manufacturers’ instructions. A portion of the culture medium was treated with an equal volume of LDH substrate solution for 30 minutes and then stopped with 5 volumes of 0.1 M NaOH; a spectrophotometer was used to measure the absorbance at 440 nm in sister cultures that were treated with 1/100 volume of 10% Triton X-100 and incubated for 30 minutes at 37°C.
Caspase-3 and caspase-9 activity assays
We measured the cleavage of chromogenic caspase substrates, Ac-DEVD-pNA (acetyl-Asp-Glu-Val-Asp p-nitroanilide), a caspase-3 substrate, and Ac-LEHD-pNA (acetyl-Leu-Glu-His-Asp p-nitroanilide), a caspase-9 substrate, to calculate caspase-3 and caspase-9 activities, respectively. The experiment was performed according to the manufacturer’s protocols. Approximately 50 µg of total protein was added to the reaction buffer containing Ac-DEVD-pNA (2 mM) or Ac-LEHD-pNA (2 mM) and then incubated at 37°C for 2 hours. The absorbance of yellow pNA cleaved from its corresponding precursors was measured using a spectrometer at 405 nm. The specific caspase activities, normalized to the total protein of the cell lysates, were then expressed as the fold change relative to the baseline of control cells cultured in DMEM with 10% FBS.
Western blot assay
We used protein lysis buffer (Beyotime Institute of Biotechnology) together with the appropriate concentrations of proteinase inhibitors and phosphatase inhibitors (Roche Applied Science, Indianapolis, IN, USA) to extract cell proteins. Then, the Bradford assay (Beyotime Institute of Biotechnology) was used to detect the total protein concentration. The protein samples were fractionated by SDS-PAGE (12% polyacrylamide gels) followed by transfer onto nitrocellulose membranes (Bio-Rad Laboratories Inc., Hercules, CA, USA). After incubation in blocking buffer (20 mM Tris, pH 7.6, 150 mM NaCl, and 0.1% Tween-20) containing 5% nonfat dry milk powder, the membranes were incubated with the indicated antibodies overnight at 4°C, followed by reaction with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 hour at room temperature. Enhanced chemiluminescent reagents were used to visualize the protein bands by chemiluminescence detection kit. Beta-actin was used as an internal control.
Statistical analyses
The composite data were expressed as mean±standard error of the mean (SEM) of at least three independent experiments. Statistical analyses were performed by Student’s t-test or one-way ANOVA followed by Dunnett’s test where appropriate. P<0.05 was considered statistically significant.
Results
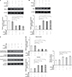
12-HETE inhibits cell apoptosis in a dose-dependent manner
It is well known that an increased resistance to apoptosis is a hallmark of cancer. To determine the crucial role of 12-HETE in the growth of ovarian cancer cells, we examined whether 12-HETE protected against cell apoptosis in ovarian cancer cells. In this study, SD was used as an apoptotic model for experiments. As shown in Figure 1A, the MTT results showed that cell viability was significantly decreased by SD after 48 hours of treatment, while SD-induced decreases in cell viability were obviously mitigated by 1 µM exogeneous 12-HETE. We obtained similar results when we detected the activity of caspase-3. The results show that 1 µM 12-HETE significantly decreases the activation of caspase-3 induced by SD (Figure 1B). Moreover, we also examined cellular half maximal inhibitory concentration (IC50) values of 12-HETE for the caspase-3 activity in OVCAR-3 cells and found that 12-HETE repressed the increased activity of caspase-3 induced by SD in a concentration-dependent manner, with an IC50 value of 1.13 µM (Figure 1C). The results indicate that 12-HETE protects against cell apoptosis in ovarian cancer cells in a concentration-dependent manner.
The effects of 12-HETE on cell apoptosis are mediated by the ILK pathway
Our previous study demonstrated that ILK participates in the regulation of the progression of ovarian cancer and plays a critical role in the survival of cancer cells.15 We examined whether 12-HETE was responsible for the activation of the ILK pathway during the progression of ovarian cancer. In OVCAR-3 cells, treatment with 1 µM 12-HETE led to the increased expression of ILK (Figure 2A). To elucidate the role of ILK in the inhibition of cell apoptosis by 12-HETE, ILK siRNA (siILK) was used to knock down the expression of ILK (Figure 2B). As shown in Figure S1, cell viability, caspase-3 activity, and ILK expression were not obviously affected by the transfection of siControl compared with the untransfected OVCAR-3 cells (Figure S1A–C), implying that there are no significant differences between the siControl transfection and untransfected cells. Thus, we utilized the siControl transfection cells as the negative control in the following experiments. The results of a cell growth assay showed that 1 µM 12-HETE antagonized the decrease in cell viability induced by SD, but the effects of 12-HETE on cell survival were abolished after repressing the expression of ILK with siILK (Figure 2C). As shown in Figure 2D, the release of LDH (an indication of cell death) induced by SD was attenuated by 1 µM 12-HETE, whereas the ILK siRNA eliminated the roles of 12-HETE in LDH release (Figure 2D). Moreover, the inhibitory effects of 12-HETE on the activation of caspase-3 and the increased expression of cleaved caspase-3 induced by SD were attenuated by ILK siRNA (Figure 2E and F). These results imply that 12-HETE promotes the survival of ovarian cancer cells by activating the ILK pathway.
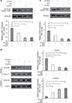
12-HETE activation of the ILK pathway inhibits mitochondria-dependent apoptosis
Intrinsic cell apoptosis is an important event induced by SD that leads to cell growth arrest. We examined some important changes in mitochondria-dependent apoptosis, including the protein levels of Bcl-2 and Bax, the release of cytochrome C into the cytoplasm, and the activity of caspase-9. As shown in Figure 3A and B, 1 μM 12-HETE induced the expression of Bcl-2 (an antiapoptotic protein) and depressed the expression of Bax (a pro-apoptotic protein), and these effects were weakened by the ILK siRNA. Moreover, the release of cytochrome C from the mitochondria into the cytoplasm is a critical event that triggers the progression of mitochondria-dependent apoptosis. We found that SD treatment led to increased expression of cytoplasmic cytochrome C and decreased expression of cytochrome C in mitochondria, but 1 µM 12-HETE mitigated the release of cytochrome C from the mitochondria into the cytoplasm. However, the inhibitory effects of 12-HETE on cytochrome C release were eliminated by the ILK siRNA (Figure 3C). In addition, we also examined the activity of caspase-9, which is specifically activated in the intrinsic apoptosis pathway. We found that 1 µM 12-HETE inhibited the activation of caspase-9 induced by SD, while the ILK siRNA impaired the inhibitory effects of 12-HETE on caspase-9 activation (Figure 3D). These results imply that ILK is involved in the inhibition of mitochondria-dependent apoptosis by 12-HETE.
Meanwhile, to validate the role of 12-HETE in cell apoptosis, we also tried an additional concentration of 12-HETE for some functional experiments. Our results showed that the treatment with 3 µM 12-HETE induced the expression of ILK in OVCAR-3 cells (Figure S2A). Both the decreased cell viability and the increased LDH release induced by SD were mitigated by 3 µM 12-HETE. However, the ILK siRNA antagonized the facilitating roles of 12-HETE in cell survival (Figure S2B and C). Moreover, the treatment with 3 µM 12-HETE inhibited the activation of caspase-3 and increased the expression of Bax induced by SD, but these effects were eliminated by ILK siRNA (Figure S2D and E).
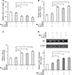
The activation of NF-κB induced by 12-HETE is eliminated by silencing the expression of ILK
NF-κB is an ubiquitous transcription factor the dysfunction of which is closely related to different types of cancers. The increased activation of NF-κB is a predictor of poor disease progression and confers resistance to cell apoptosis.16 Based on this evidence, we examined the status of NF-κB in ovarian cancer cells under conditions of 12-HETE treatment. The results showed that 1 µM 12-HETE led to increased levels of NF-κB p65 phosphorylation, and the increased phosphorylation of NF-κB p65 caused by 12-HETE was attenuated by the knockdown of ILK (Figure 4A). Moreover, the protein levels of NF-κB p65 in cytoplasmic and nuclear extracts indicated that 1 µM 12-HETE treatment caused a significant increase in the protein levels of nuclear NF-κB p65, which was accompanied by decreased levels of NF-κB p65 in the cytoplasm. However, the nuclear translocation elicited by 12-HETE treatment was abolished by the ILK siRNA (Figure 4B). In addition, the activation of NF-κB is closely modulated by IKB kinases. Hence, we further examined the effects of 12-HETE on the phosphorylation of IKBa protein. As shown in Figure 4C, 1 μM 12-HETE promoted the phosphorylation of IKBa, which was attenuated by the ILK siRNA. These results indicate that 12-HETE facilitates the activation and nuclear translocation of NF-κB via ILK in ovarian cancer cells.
In addition, to avoid the nonspecific suppression of the ILK siRNA, we then utilized another independent siRNA of ILK (siILK#2) and repeated some important experiments. The knockdown efficiency of siILK#2 was confirmed by Western blot (Figure S3A). We found that the inhibitory effects of 1 µM 12-HETE on the decrease of cell viability and the increase of caspase-3 activity induced by SD were mitigated by siILK#2 (Figure S3B and C). Moreover, 12-HETE repressed the expression of Bax and increased the phosphorylation of NF-κB p65, but these effects were abolished after repressing the expression of ILK with siILK#2 (Figure S3D and E).
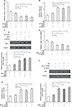
The inhibitory effects of 12-HETE on cell apoptosis are weakened by the inhibition of NF-κB
To illustrate the critical role of NF-κB in the inhibition of cell apoptosis by 12-HETE, we used 5 µM Bay-117082 to block the NF-κB pathway in ovarian cancer cells.17 As shown in Figure 5A, the cell viability increase caused by 1 µM 12-HETE was antagonized by the NF-κB inhibitor. In addition, the inhibitory effects of 12-HETE on SD-induced activation of caspase-3 and caspase-9 were weakened by the inhibition of NF-κB (Figure 5B and C). Moreover, the 12-HETE-mediated decrease of the protein level of Bax was mitigated by Bay-117082 (Figure 5D). These results indicate that 12-HETE protects against cell apoptosis through the ILK/NF-κB pathway in ovarian cancer.
12-HETE inhibited cell growth and apoptosis via ILK and NF-κB in SKOV3 ovarian cancer cells
To determine whether the anti-apoptotic effect of 12-HETE is specific to OVCAR-3 cells, we examined its roles in SKOV3 ovarian cancer cells. Our results showed that 1 µM 12-HETE inhibited the decrease in cell viability induced by SD and that this effect was attenuated by siILK (Figure 6A). The activation of caspase-3 and increased protein level of Bax were repressed in SKOV3 cells by treatment with 1 µM 12-HETE, while the inhibitory effects of 12-HETE on cellular apoptosis were mitigated by knockdown of ILK (Figure 6B and C). Moreover, 1 µM 12-HETE inhibited the decrease in cell viability, and the 12-HETE-mediated increase in caspase-3 activity and Bax expression was impeded by an inhibitor of the NF-κB pathway (Figure 6D–F). All of the above results imply that 12-HETE promotes cell survival by regulating the ILK/NF-κB pathway in ovarian cancer cells.
Discussion
Ovarian cancer is a common gynecologic malignancy in the female reproductive system.1 However, the molecular mechanisms of ovarian cancer progression were imperfectly understood until now. In the present study, our results demonstrate an important role for 12-HETE in regulating the survival of ovarian cancer cells and illustrate the corresponding molecular mechanism involved.
Apoptosis plays a negative role in the development of cancers. An important step in the progression of tumors is the overgrowth of cancer cells, and the increased resistance of cancer cells to apoptosis strongly contributes to their infinite growth. Moreover, genes involved in modulating cell apoptosis are considered to be a class of tumor-related genes.18 Previous reports have shown that the 12-LOX/12-HETE pathway is necessary for cell survival in vascular smooth muscle cells and that the expression of 12-LOX and the generation of 12-HETE are upregulated in different types of cancers.8,19 However, no systematic study has revealed the corresponding molecular mechanism of 12-HETE in modulating ovarian cancer cell apoptosis. In this study, our results showed that 12-HETE inhibited cell apoptosis and promoted cell growth in a dose-dependent manner.
ILK, a serine-threonine kinase, has been implicated in the activation of a range of signaling pathways that regulate cell growth, survival, and tumor angiogenesis.11 It has been reported that the upregulation of ILK strongly correlates with the invasion and progression of some types of cancers, and increased expression of ILK has been found in the serum and tumor tissues of patients with cancer.20,21 In our research, we first demonstrate that 12-HETE, an important metabolic product of AA catalysis by 12-LOX, acts as a critical upstream regulator of ILK. We found that 12-HETE treatment induced the expression of ILK in ovarian cancer cells. Moreover, the inhibitory effects of 12-HETE on cytochrome c release into the cytoplasm and activation of caspase-9 and caspase-3 were abolished by the knockdown of ILK. The results indicate that 12-HETE inhibits cell apoptosis by upregulating ILK in ovarian cancer.
The transcription factor NF-κB, a major inducer of stress-response genes in the survival pathway, is associated with many biological processes, including inflammation, angiogenesis, cell proliferation, and apoptosis.22 The NF-κB family has been demonstrated to participate in the regulation of cancer progression. It has been reported that the increased activity of NF-κB is significantly correlated with cancer progression and poor patient prognosis, and the resistance to apoptosis was conferred to ovarian cancer cells by activation of NF-κB.16 An important step for activating the biological activity of NF-κB is the phosphorylation of NF-κB p65.23 In the present study, we found that 12-HETE treatment augmented the phosphorylation of NF-κB p65 and promoted the nuclear translocation of NF-κB p65, but this effect was attenuated by knockdown of ILK. Moreover, IkBa, a protein known to phosphorylate the transactivation domain of NF-κB p65, modulates the activation of NF-κB.24,25 Our results showed that the 12-HETE-enhanced phosphorylation of IkBa was abolished by ILK siRNA. These results indicate that the increases in the activation and nuclear translocation of NF-κB induced by 12-HETE are mediated by ILK.
Conclusion
Our study suggests that 12-HETE inhibits the mitochondria-dependent apoptosis pathway through the ILK pathway and that NF-κB acts as a downstream target of 12-HETE-activated ILK. This study implies that 12-LOX/12-HETE and its downstream targets (ILK and NF-κB) play critical roles in promoting the survival of ovarian cancer cells and positively regulate the progression of ovarian cancer, which indicates a new potential target for future treatment of ovarian cancer.
Acknowledgment
This work was supported by grants from National Natural Science Youth Foundation of China (No. 81401502), Nature Scientific Foundation of Heilongjiang Province (No. H2018020), Heilongjiang Postdoctoral Foundation and Health Department Foundation of Heilongjiang Province (No. 2010–097).
Disclosure
The authors report no conflicts of interest in this work.
References
Landen CN, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26(6):995–1005. | ||
Vergote I, Tropé CG, Amant F. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. | ||
Hedström E, Pederiva C, Farnebo J, et al. Downregulation of the cancer susceptibility protein WRAP53β in epithelial ovarian cancer leads to defective DNA repair and poor clinical outcome. Cell Death Dis. 2015;6:e1892. | ||
Liu JY, Yang J, Inceoglu B, et al. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010;79(6):880–887. | ||
Hu Y, Sun H, O’Flaherty JT, Edwards IJ. 15-Lipoxygenase-1-mediated metabolism of docosahexaenoic acid is required for syndecan-1 signaling and apoptosis in prostate cancer cells. Carcinogenesis. 2013;34(1):176–182. | ||
Ara G, Teicher BA. Cyclooxygenase and lipoxygenase inhibitors in cancer therapy. Prostaglandins Leukot Essent Fatty Acids. 1996;54(1):3–16. | ||
Jiang Y, Pan Y, Rhea PR, et al. A Sucrose-Enriched Diet Promotes Tumorigenesis in Mammary Gland in Part through the 12-Lipoxygenase Pathway. Cancer Res. 2016;76(1):24–29. | ||
Chang J, Jiang L, Wang Y, et al. 12/15 Lipoxygenase regulation of colorectal tumorigenesis is determined by the relative tumor levels of its metabolite 12-HETE and 13-HODE in animal models. Oncotarget. 2015;6(5):2879–2888. | ||
Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999;9(8):319–323. | ||
Dedhar S. Cell-substrate interactions and signaling through ILK. Curr Opin Cell Biol. 2000;12(2):250–256. | ||
Han KS, Li N, Raven PA, et al. Targeting Integrin-Linked Kinase Suppresses Invasion and Metastasis through Downregulation of Epithelial-to-Mesenchymal Transition in Renal Cell Carcinoma. Mol Cancer Ther. 2015;14(4):1024–1034. | ||
Choi YP, Kim BG, Gao MQ, Kang S, Cho NH. Targeting ILK and β4 integrin abrogates the invasive potential of ovarian cancer. Biochem Biophys Res Commun. 2012;427(3):642–648. | ||
Li Q, Li C, Zhang YY, et al. Silencing of integrin-linked kinase suppresses in vivo tumorigenesis of human ovarian carcinoma cells. Mol Med Rep. 2013;7(3):1050–1054. | ||
Lössner D, Abou-Ajram C, Benge A, Aumercier M, Schmitt M, Reuning U. Integrin alphavbeta3 upregulates integrin-linked kinase expression in human ovarian cancer cells via enhancement of ILK gene transcription. J Cell Physiol. 2009;220(2):367–375. | ||
Liu Q, Xiao L, Yuan D, Shi X, Li P. Silencing of the integrin-linked kinase gene induces the apoptosis in ovarian carcinoma. J Recept Signal Transduct Res. 2012;32(2):120–127. | ||
Annunziata CM, Stavnes HT, Kleinberg L, et al. Nuclear factor kappaB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer. 2010;116(13):3276–3284. | ||
Singha B, Gatla HR, Manna S, et al. Proteasome inhibition increases recruitment of IκB kinase β (IKKβ), S536P-p65, and transcription factor EGR1 to interleukin-8 (IL-8) promoter, resulting in increased IL-8 production in ovarian cancer cells. J Biol Chem. 2014;289(5):2687–2700. | ||
Ryoo HD, Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb Perspect Biol. 2012;4(8):a008797. | ||
Yang P, Cartwright CA, Li J, et al. Arachidonic acid metabolism in human prostate cancer. Int J Oncol. 2012;41(4):1495–1503. | ||
Dai DL, Makretsov N, Campos EI, et al. Increased expression of integrin-linked kinase is correlated with melanoma progression and poor patient survival. Clin Cancer Res. 2003;9(12):4409–4414. | ||
Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5(1):51–63. | ||
Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–208. | ||
Kwon HJ, Choi GE, Ryu S, et al. Stepwise phosphorylation of p65 promotes NF-κB activation and NK cell responses during target cell recognition. Nat Commun. 2016;7:11686. | ||
Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274(43):30353–30356. | ||
Cohen L, Henzel WJ, Baeuerle PA. IKAP is a scaffold protein of the IkappaB kinase complex. Nature. 1998;395(6699):292–296. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.