Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
(–)-Epigallocatechin-3-Gallate Protects Human Skin Fibroblasts from Ultraviolet a Induced Photoaging
Authors Jia Y, Mao Q, Yang J, Du N, Zhu Y , Min W
Received 29 November 2022
Accepted for publication 10 January 2023
Published 19 January 2023 Volume 2023:16 Pages 149—159
DOI https://doi.org/10.2147/CCID.S398547
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yuanyuan Jia,1 Qiuyu Mao,1 Jingyi Yang,1 Na Du,1 Yuan Zhu,2 Wei Min1
1First Affiliated Hospital of Soochow University, Suzhou, Jiangsu Province, People’s Republic of China; 2First People’s Hospital of Changshu City, Changshu Hospital Affiliated of Soochow University, Changshu, Jiangsu Province, People’s Republic of China
Correspondence: Wei Min, Email [email protected]
Background: Ultraviolet (UV) is a common stressor of skin and repeated UVA radiation contributes to photoaging. (–)-Epigallocatechin-3-Gallate (EGCG), as the major polyphenol that is found in green tea, and catechins and have shown considerable antioxidant capacity.
Purpose: Our study aims to explore the effects of EGCG on UVA-induced skin photoaging process and associated mechanisms.
Methods: In this study, human skin fibroblasts (HSFs) were treated with UVA and EGCG, and subsequent changes in cell morphology, telomeres, antioxidant capacity, cell cycle, and related genes were evaluated to examine the role and mechanisms of EGCG in delaying skin photoaging.
Results: HSF exposed to UVA underwent an increase in aging-related biomarkers and telomere shortening. Also, UVA radiation inhibited the secretion of transforming growth factor-beta1 (TGF-β 1), induced cell cycle arrest, down-regulated antioxidant enzymes, and promoted the accumulation of oxidative product malondialdehyde (MDA) to cause further damage to cells. Increased expression of matrix metalloproteinases (MMPs), tissue inhibitor of metalloproteinase-1 (TIMP-1), p66 at mRNA levels were also observed after UVA irradiation. EGCG treatment effectively inhibited above damage processes caused by UVA radiation in HSF.
Conclusion: Our study indicated that the potential mechanism of EGCG retarding photoaging is closely related to its powerful antioxidant effects and the ability to regulate the expression of related genes, and the usage of EGCG will be a potential strategy in preventing skin photoaging induced by UVA radiation.
Keywords: ultraviolet A, human skin fibroblasts, (–)-Epigallocatechin-3-Gallate, EGCG, photoaging
Introduction
Long-term repeated exposure to ultraviolet (UV) is the most important factor that causes skin senescence and this phenomenon is called photoaging. UV, with a wavelength of 10nm to 400nm, is invisible light outside the purple light in the spectrum and is divided into UVA, UVB, and UVC. UVC (200 ~ 280nm, short wave) has the weakest penetration, which can almost be completely absorbed by the ozone layer, which effects on skin can be ignored. The largest amount of UVB (280 ~ 320nm, mediumwave) penetrates into the epidermis and cannot enter the deep part of the skin. The penetration of UVA (320–400nm, long-wave) far exceeds that of UVB, reaching the basal and dermal layers of the skin and causing chronic damage to collagen, elastic fibers, and proteoglycans.1–3 Frequent exposure to UVA causes skin inflammation, erythema, wrinkles, and cancelation. Photoaging is manifested by changes in tissue structure of the skin, but the most characteristic variation is in dermal, resulting from the degeneration, degradation, and abnormal aggregation of elastic fibers and collagen fibers, as well as cleavage and increased water solubility of amino polysaccharides, leading to the appearance of sagging and deep wrinkles.4 All these components are synthesized by fibroblasts, so human skin fibroblasts (HSFs) are important targets for researching the underlying mechanisms and treatments of photoaging.
Previous studies have mainly focused on the skin damage caused by UVB because of the less carcinogenicity of UVA than UVB. However, the content of UVA in solar UV exposure is over 95% and is significantly higher than that of UVB. In addition, UVA has the ability to go deeper into the skin and reach the dermis, making the role of UVA in photoaging gradually emphasized.5,6 The mechanism by which UVA induces structural and functional changes in aging fibroblasts needs further exploration.
Catechin, a group of flavanol compounds found which are isolated from tea, has excellent antioxidant capacity. (–)-Epigallocatechin-3-Gallate (EGCG) accounts for about 50–60% of total catechin content and is where the greenest tea health benefits come from.7 A large number of studies have confirmed the effects of EGCG in resisting oxidation and cancer, preventing cardiovascular diseases and other diseases.8 Sevin et al reported that topical treatment of EGCG could reduce sunburn cell formation and dermo-epidermal harmful effects in rats, thus inhibit acute photodamage.9 It has been reported EGCG could inhibit IL-6 and TNF-α in eternal keratinocytes and reduced the UVB-induced skin photodamage and tumor formation progress.10 It has also been confirmed that EGCG could regulate UVB-induced signal transduction cascades in fibroblasts to hammer skin damage.11 Although there have been studies, which confirmed the protective effects of EGCG on skin, the effects of EGCG aging process of fibroblasts induced by UVA and relevant mechanisms remain to be fully understood.
Thus, we considered that EGCG could relieve UVA-induced photoaging in HSF. To substantiate our theory, a model of photoaging fibroblast was established to detect the role of EGCG at the cellular level. We attempted to analyze the underlying mechanisms of its photo-protection effects and provide new treatments for skin rejuvenation.
Materials and Methods
Main Reagents
We followed the materials and methods as we reported before.12 The CCC-ESF-1 human embryonic skin fibroblast cell line was obtained from the Union Medical University Cell Center. The SUV-100 solar simulator and radiant emittance monitor and EGCG (purity>95%) were obtained from Sigma Co. (Shanghai, China). A CCK-8 assay kit was purchased from Beyotime Institute of Biotech, China. The SOD, GSH-px, CAT, and MDA assay kit was from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). A reverse transcription kit and real-time quantitative PCR kit were purchased from TaKaRa Co. (Dalian, China). The cytochemical staining kit of SA-β-gal was obtained from Mirus Bio Co. (USA) and the ELISA kit for detection of extracellular Human TGF-β 1 was from R&D Co. (USA). ABI 7700 Real Time PCR System was from an Applied Biotechnology Company (USA).
Cell Culture, EGCG Treatment, and UVA Irradiation
HSF (Union Medical University Cell Center) was cultured in Dulbecco’s modified eagle’s medium (DMEM, Gibco/BRL, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Invitrogen, UK), penicillin (100 U/mL), and streptomycin (100 mg/l) at 37°C in 5% CO2. EGCG (Sigma Co, Shanghai, China) was dissolved in the culture medium. After 24 h of EGCG treatment, cells were subcultured at half confluence (1×104cells/cm2) and washed with phosphate-buffered saline (PBS) and exposed to UVA radiation. After irradiation, cultures were given fresh medium.
Cell Viability Assay
The proliferation of HSF was evaluated according to the manufacturer’s instructions. Briefly, cells were plated into a 96-well plate. After overnight incubation, the medium was cultured with EGCG for another 24 h, 48 h, and 72 h. After that, cells were incubated in the dark with CCK-8 (Beyotime Institute Biotech, China) solution for 2 h. The absorbance was measured at 450 nm with a Varioskan Flash Spectral Scanning Multimode Reader (Thermo Electron Corporation, USA).
Senescence-Associated ß-Galactosidase (SA-ß-Gal) Staining
Cells were fixed in 4% paraformaldehyde for 10 min and stained with X-gal solution for 24 h at 37°C. The population of SA-b-gal-positive (Mirus Bio Co, USA) cells was determined by counting 400 cells per dish, and images were taken with a phase-contrast microscope (Olympus, Japan).
Real-Time PCR for Telomere Length
The telomere length of fibroblasts was measured by the quantitative PCR (qPCR) technique. Genomic DNA was isolated using a genomic DNA extraction kit according to the protocol provided. The telomere-specific primer pair sequences were as follows (5’ to 3’):
for 5’-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3’,
rev 5’-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3’.
36B4 was used as the single-copy gene primers:
for 5’-CAGCAAGTGGGAAGGTGTAATCC-3’,
rev 5’-CCCATTCTATCATCAACGGGTACAA-3’.
The primers were synthesized by Shanghai Subway Gene Technology Co., LTD. The 20μL PCR reaction system was used, including SYBR Premix EX Taq 10μL, ROX Reference dye 0.4μL, each primer 0.4μL, DNA template 1μL, add DNAase-free water to 20μL, cycling for 94°C for 10 min, 5s at 95°C and 31s at 52°C for 40 cycles for telomere, or 5 s at 95°C and 31s at 54°C for 40 cycles for 36B4. The PCR data were analyzed with ABI’s SDS v.1.7 software as the Ct ie this process was done for both telomere (T) and single-copy (S) gene reactions, and telomere length was expressed as a ratio of the two, T/S ratio = 2−[Ct(telo)-Ct(36B4)] = 2−ΔCt. To determine the relative telomere length, we used the ΔCt value of UVA group as the control. The relative T/S ratio is 2−ΔΔ Ct.
TRAP-ELISA for Telomerase
Briefly, 2×105 cells per single reaction were centrifuged at 4°C for 5 min. Telomeric Repeat Amplification Protocol (TRAP) reactions were carried out according to the instructions. The absorbance was then measured at a wavelength of 450 nm in 30 min, A =A450nm-A690nm. Samples are to be considered to be positive if the difference of absorbance is higher than the 2-fold background activity.
Determination of Cell Cycle, TGF-β 1, SOD, GSH-Px, CAT, and MDA
TGF-β1 were measured with ELISA kits (R&D Co, USA) according to the manufacturer’s instructions. Absorbance was measured at 450 nm. SOD, GSH-Px, CAT, and MDA Activity Kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were assayed according to the instructions.
Cell Cycle Analysis
Cells were treated with cell cycle assays (Beyotime Institute Biotech, China) for 24, 48, and 72 h after UVA. Cells were collected and fixed with 70% cold ethanol and stored at 4°C for 24 h, and then staining with RNase at 37°C in the dark for 1 h. Cell cycle was analyzed by flow cytometry (BD FACSCalibur, USA).
Quantitative Real-Time PCR
Cellular RNA was extracted using Trizol. RNA was then used for cDNA synthesis. The reaction mixture was prepared using a SYBR Green Master Mix, and the PCR conditions were as follows: 10 min at 95°C; 45 cycles for 15s at 94°C, 20s at 59°C, and 30s at 72°C.
Sequences of primers:
hTERT for 5’-AAATGCGGCCCCTGTTTCT-3’
hTERT rev 5’-CAGTGCGTCTTGAGGAGCA-3’.
MMP-1 for 5’-CTGCTTACGAATTTGCCGAC-3’
MMP-1 rev 5’-GCAGCATC-GATATGCTTCAC-3’.
MMP-3 for 5’-CTGGACTCCGACACTCTGGA-3’
MMP-3 rev 5’-CAGGAAAGGTTCTGAAGTGACC-3’.
MMP-10 for 5’-TGCTCTGCCTATCCTCTGAGT-3’,
MMP-10 rev 5’-TCACATCCTTTTCGAGGTTGTAG-3’.
TIMP-1 for 5’-GATGGACTCTTGCACATCACT-3’
TIMP-1 rev 5’-TGGATAAACAGGGAAACACTG-3’.
p66Shc for 5’-ACTACCCTGTGTTCCTTCTTTC-3’
p66Shc rev 5’-GCAGCATCGATATGCTTCAC-3’.
Statistical Analysis
SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Data are presented as mean ± standard deviation. The data were analyzed with ANOVA followed by an LSD test for individual comparisons between group means. Statistical analysis of P < 0.05 was considered significant.
Results
Effects of EGCG and UVA Irradiation on Fibroblasts Viability
We assessed its cytotoxic effect at increasing concentrations ranging from 0 to 40μg/mL to determine the appropriate EGCG concentration. No inhibitory effect of EGCG on HSF cell viability was observed, and the proliferation activity of HSF cultured with EGCG was the highest at the concentration of 25μg/mL (Figure 1A). Therefore, we selected 25μg/mL for a period of 24 h treatment for further mechanism studies. HSFs were treated with 10, 20 and 30J/cm2 UVA for 2 weeks. It can be observed that cell proliferation activity in the UVA irradiation group had a significant reduction compared with the control group, fell by 17%, 42% and 51%, respectively, and the proliferation activity decreased with the increase in UVA irradiation dose (Figure 1B). 20J/cm2 and 30Jcm2 UVA irradiation had a strong cytotoxic effect on cells. HSF irradiated with 10J/cm2 UVA showed the least toxic reaction with the proliferation activity decreased. Therefore, we determined that 10J/cm2 is the most appropriate sub-cytotoxic UVA irradiation dose for a follow-up study.
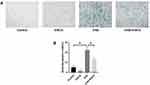
EGCG Reversed SA-β-Gal Activity Induced by UVA
Our results further showed that EGCG could reduce proportion of aging cells (Figure 2A). As expected, UVA irradiation strongly induced an increase of positively stained HSF (23.07%) and treated with EGCG significantly suppressed the UVA-induced expression of SA-β-gal to 12.75%, demonstrating that EGCG could slow down the process of photoaging to some extent (Figure 2B).
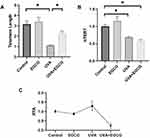
EGCG Protects Telomeres from UVA Damage
Previous researches showed that cell senescence is closely related to telomere shortening because telomere replication is incomplete and would lose some fragments as cells divide. Ageing human cells will stop dividing when the telomere length is shortened to some extent. Our study indicated that the telomeres of the EGCG group were not significantly prolonged and telomeres of the HSF irradiated with UVA were shortened. The relative telomere length of normal HSF was about 3.16±0.31, significantly longer than that in the UVA irradiation group. The telomere length treated with EGCG was 3.40±0.42, which showed no further lengthening tendency compared to the control cells. Besides, it can be observed that the telomere length in EGCG and UVA group is 2.25 ± 0.24, which was increased in comparison to the UVA radiation group (Figure 3A). We also inspect the expression of the telomerase catalyzed subunit gene called human telomerase reverse transcriptase (hTERT) and the tartrate-resistant acid phosphatase (TRAP)-ELISA telomerase detection kit was applied to analyze the relative telomerase activity (RTA), both of which would reflect telomerase activity in HSF. UVA irradiation significantly decreased the expression of hTERT (Figure 3B). EGCG could not significantly regulate the expression of hTERT. Meanwhile, it could be observed that EGCG could significantly protect cells from telomerase activity (Figure 3C).
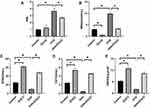
EGCG Counteracted UVA-Induced Cell Cycle Arrest
UV irradiation could induce G1 phase arrest, leading to senescence. Cell populations in the G1 phase increased to 81.04% from 59.94% in the control group. The proportion of G1 phase cells increased slowly after different doses of UVA, rising to 89.09% after 72 h of irradiation. However, the ratio of G1 phase HSF with EGCG was 77.71% 24 h after radiation, suggesting that EGCG had a certain effect on the UVA-induced cell cycle block (Figure 4A and B).
EGCG Inhibited UVA-Induced Oxidation Reaction
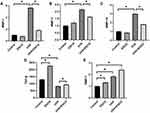
Long-term accumulative of damage and oxidative reactions caused by UVA irradiation accelerate the process of photoaging, leading to the consumption of antioxidant substances and the accumulation of oxidative products. There is also evidence that p66Shc up-regulation was associated with oxidative stress. In our study, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-px) activities in HSF were significantly decreased with UV group, while levels of malondialdehyde (MDA) and P66Shc in irradiated cells were significantly increased, indicating that UVA irradiation reduced antioxidant ability of HSF and caused oxidative damage. Meanwhile, the content of SOD, GSH-px and CAT in the HSF treated with EGCG alone increased remarkably, while the generation of MDA and P66 was inhibited, and the pre-addition of EGCG significantly inhibited UVA-induced MDA production and antioxidant suppression (3.34±0.4) (Figure 5A–E).
EGCG Promotes Collagen Synthesis and Inhibits Collagen Degradation Caused by UVA
Transforming growth factor-beta1 (TGF-β1) stimulates the proliferation of HSF and accelerates the synthesis of extracellular matrix such as collagen, which is the strongest pro-fibrotic cytokine ever known. Nevertheless, the activity of TGF-β1 is weakened in photoaging skin. Our study found that in the absence of UVA irradiation, TGF-β1 secreted by cells in the EGCG-treated group increased to 2270.4±42.53 pg/mL. Irradiation of UVA reduced the secretion level of TGF-β1 in HSF to 818.97±37.99 pg/mL, which was significantly alleviated to 934.88±27.61pg/mL in the radiation group pre-treated with EGCG (Figure 6D). It was also conducted to explore whether EGCG could protect cells from UVA-induced matrix metalloproteinases (MMPs) production. As expected, UVA irradiation elevated the MMPs level (Figure 6A–C), while EGCG protected cells from the destruction of these enzymes. Consistently, there was also a significant increase in the mRNA level of tissue inhibitor of metalloproteinase-1 (TIMP-1) and TGF-β with EGCG, which played key roles in the extracellular matrix (Figure 6D and E). These observations implied that EGCG functioned as an anti-photoaging agent.
Discussion
It has been reported Senescence-related β-galactosidase (SA-β-gal) increased with the growth of cell age, which can be used to evaluate cell aging. Our results suggested that the cells did enter the photoaging stage with UVA. Meanwhile, fibroblasts pre-treated with EGCG experienced a significant decrease in the proportion of aging cells, indicating that EGCG could alleviate fibroblasts photoaging.
Telomeres, which are located at the ends of eukaryotic chromosomes, maintain chromosomal stability while ensuring that cells divide continuously.13 However, the telomere itself cannot be completely replicated because of the “end replication problem of linear chromosomes”, and once the Hayflick limit is reached during the progress of DNA lost, the cell enters an aging state and quits dividing. Telomerase is a DNA polymerase present in eukaryotic cells. To protect telomeres length and escape from cellular senescence in rapidly dividing cells, telomerase utilizes RNA components complementary to telomere repeats as primers to elongate telomeres. Telomerase activity is closely related to human telomerase reverse transcriptase (hTERT) expression, while normal human somatic cells rarely exhibit telomerase activity. UV causes DNA to form pyrimidine dimers directly or indirectly and destroys the stability of DNA through reactive oxygen species (ROS)-mediated mechanisms.14 The telomere is rich in guanine and thymine due to its conserved sequence and 3’ terminal structure, making it more prone to oxidative damage caused by cell metabolism or ultraviolet. This is also the reason why telomere shortening in photoaged cells is faster than that of normal aging cells.4 In this study, the telomere of cells irradiated with UVA was significantly shortened and the addition of EGCG helped limit the shortening caused by UVA radiation, indicating that EGCG can delay telomere shortening and attenuated photoaging. Considering the possibility that EGCG could activate telomerase and protected telomeric DNA, we examined the changes of hTERT to further reveal the mechanism by which EGCG protected telomere and enhanced cell replication ability and confirmed that the activity of fibroblast telomerase and the intensity of hTERT expression did not significantly increase under the intervention of EGCG.15 Thus, we speculated that the effects of EGCG to maintain telomere and promote cell proliferation are probably correlated with its antioxidant properties and ability to clearance free radicals, other than telomerase.
Ultraviolet mediated cyclobutane pyrimidine dimers (CPDs) formation destroyed the leads to cell death.16 Our study suggested that UVA could induce G1-phase cell cycle arrest to alleviate further DNA damage and minimize the possibility of gene mutations, in the early stage of UVA-induced senescence, which may lead to stable cell cycle arrest and senescence if not intervened. In this study, treatment with EGCG significantly alleviated the increase of G1-phase cells and reduced senescence caused by cell cycle arrest.
To deeply analyze the possible mechanism of the EGCG photo-protective effects on HSF, extracellular TGF-β1 was examined by collecting and testing the supernatant of cultured HSF. Studies have shown that TGF-β1, as the strongest known pro-fibrotic factor, promoted the proliferation of fibroblasts, stimulated fibroblasts to synthesize extracellular matrix (ECM) and inhibited the activity of proteases and matrix enzymes, thereby depositing ECM and maintaining the appearance of young, smooth skin. Fisher et al and Quan et al found that the level of autocrine TGF-β1 reduced and cell response to TGF-β1 decreased in photoaged skin.17,18 The TGF-β1 receptor on the cell surface decreased and the phosphorylation reaction after ligand-receptor binding was weakened as aging progressed, which resulted in the inhibition of fibroblasts function and the acceleration of skin photoaging. In our study, we observed that TGF-β1 in the supernatant of photoaged cells was significantly reduced and EGCG brought it back to a relatively normal level. Based on this, we reasoned that the anti-aging mechanism of EGCG was achieved by stimulating HSF to secrete TGF-β1. Clinically, photoaging is primarily characterized by loose rough skin, leather-like wrinkles, and adermotrophia. The root cause of these symptoms is the degradation and messy arrangement of collagen and ECM components after long-term repeated ultraviolet radiation. Studies have confirmed that Ultraviolet stimulates mitogen-activated protein kinase (MAPK) signal transduction pathway, external signal-regulated kinase (ERK) pathway and stress-activated protein kinase (SAPK) pathway by activating relevant cytokine receptors in fibroblasts, such as EGFR, IL1-R, TNF-R, etc., ultimately synthesizing transcription factor activator protein 1 (AP-1) and inducing the generation of MMPs, which hydrolyze collagen and matrix within the dermis and contribute to photoaging.19–21 TIMPs are a group of secreted glycoproteins that attenuate MMPs activity.22 The balance between TIMPs and MMPs provides a suitable environment for the construction and maintenance of skin tissue. Our results showed that the expression of MMPs of photoaged HSF was significantly increased, which was reduced by EGCG pretreatment. Treatment with EGCG or UVA radiation alone increased the mRNA expression level of TIMP, while the TIMP mRNA expression level of cells with both UVA and EGCG treatment experienced a further increase. Our results suggested that UVA induced the synthesis of MMPs, while the skin would initiate a self-protection mechanism to synthesize TIMP-1 to antagonize the effects of MMPs, and EGCG helped to degrade MMPs and accelerated the synthesis of TIMP-1, reducing collagen degradation and returning dermis to its ordered structure.
Oxidative stress reaction occurs when the skin is irradiated with UV, which promotes the production of large amounts of ROS. This is mainly mediated by the adaptor protein p66Shc. Skin tissue metabolizes vigorously and oxidative reactions are more likely to occur, thus p66Shc is highly expressed and exists in both cytoplasm and mitochondria.23 P66Shc transfers oxidative stress signals to mitochondria when cells are oxidatively damaged, up-regulated ROS production, and motivated signal transduction. We found that once irradiated with UVA, the mRNA expression level of p66Shc in HSF increased sharply, while the expression of p66Shc in irradiated HSF pretreated with EGCG had a significant decrease, suggesting that p66Shc was involved in UVA-induced photoaging and EGCG can alleviate oxidative damage and cell apoptosis through mechanisms related to p66Shc. Excessively generated ROS also breaks the balance between oxidation and antioxidant reaction, which directly damage the lipids, proteins, and nucleic acids of cells.24 Present studies have also suggested that inhibition of hTERT transcription factors was regulated through a ROS-dependent pathway, and overexpression of antioxidant enzymes mitigated antitelomerase activity, suggesting a possible mechanism between antioxidants and telomerase function after receiving EGCG treatment.25 Cellular antioxidant enzymes can also exert protective effects by regulating the synthesis and degradation of collagen. ROS accumulation can induce the production of MMPs, while antioxidants can alleviate the degradation of extracellular matrix in photoaging skin.26,27 Our study confirmed that EGCG reduced ROS generation and protected the activities of antioxidant enzymes including SOD, GSH-px, CAT, and reduced the production of MDA as well. It was also reported that after catechin treatment in mice, the expression of antioxidant enzymes regulated by Nrf2/keap1 in mice increased, such as Glutamate cysteine ligase (GCL) and Heme oxygenase-1 (HO-1).28,29 These data clarified that EGCG exerted its powerful antioxidant capacity and maintained cell vitality in multiple ways.
In conclusion, we demonstrate that EGCG delayed UVA-induced photoaging of dermal fibroblasts, which is related to its regulation of TGF β-1, P66Shc, cell redox system, MMPs and TIMP-1 and delaying telomere shortening, which makes EGCG a more attractive target in the prevention and treatment of photoaging.
Ethics Approval and Informed Consent
The research involving human participants was reviewed and approved by the Medical Ethics Committee of the Affiliated Hospital of Soochow University. The participants provided their written informed consent to participate in this study. The research was carried out following the Helsinki Declaration.
Acknowledgments
This research was funded by Jiangsu Commissions of Health Program (H2019050) and Jiangsu Provincial Administration of Traditional Chinese Medicine (MS2021099).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Jiangsu Commissions of Health Program (H2019050) and Jiangsu Provincial Administration of Traditional Chinese Medicine (MS2021099).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dupont E, Gomez J, Bilodeau D. Beyond UV radiation: a skin under challenge. Int J Cosmet Sci. 2013;35(3):224–232. doi:10.1111/ics.12036
2. Seo SW, Park SK, Oh SJ, Shin OS. TLR4-mediated activation of the ERK pathway following UVA irradiation contributes to increased cytokine and MMP expression in senescent human dermal fibroblasts. PLoS One. 2018;13(8):e0202323. doi:10.1371/journal.pone.0202323
3. Chen X, Li L, Xu S, et al. Ultraviolet B radiation down-regulates ULK1 and ATG7 expression and impairs the autophagy response in human keratinocytes. J Photochem Photobiol B. 2018;178:152–164. doi:10.1016/j.jphotobiol.2017.08.043
4. Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol. 2007;157(5):874–887. doi:10.1111/j.1365-2133.2007.08108.x
5. Bachelor MA, Bowden GT. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin Cancer Biol. 2004;14(2):131–138. doi:10.1016/j.semcancer.2003.09.017
6. Battie C, Jitsukawa S, Bernerd F, Del Bino S, Marionnet C, Verschoore M. New insights in photoaging, UVA induced damage and skin types. Exp Dermatol. 2014;23(Suppl 1):7–12. doi:10.1111/exd.12388
7. Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67(17):1849–1855. doi:10.1016/j.phytochem.2006.06.020
8. Devika PT, Stanely Mainzen Prince P. Protective effect of (-)-epigallocatechin-gallate (EGCG) on lipid peroxide metabolism in isoproterenol induced myocardial infarction in male Wistar rats: a histopathological study. Biomed Pharmacother. 2008;62(10):701–708. doi:10.1016/j.biopha.2007.10.011
9. Sevin A, Oztaş P, Senen D, et al. Effects of polyphenols on skin damage due to ultraviolet A rays: an experimental study on rats. J Eur Acad Dermatol Venereol. 2007;21(5):650–656. doi:10.1111/j.1468-3083.2006.02045.x
10. Luo D, Min W, Lin XF, Wu D, Xu Y, Miao X. Effect of epigallocatechin gallate on ultraviolet B-induced photo-damage in keratinocyte cell line. Am J Chin Med. 2006;34(5):911–922. doi:10.1142/S0192415X06004387
11. Bae JY, Choi JS, Choi YJ, et al. (-)Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: involvement of mitogen-activated protein kinase. Food Chem Toxicol. 2008;46(4):1298–1307. doi:10.1016/j.fct.2007.09.112
12. Min W, Liu X, Qian Q, et al. Effects of baicalin against UVA-induced photoaging in skin fibroblasts. Am J Chin Med. 2014;42(3):709–727. doi:10.1142/S0192415X14500463
13. Doksani Y, Wu JY, de Lange T, Zhuang X. Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell. 2013;155(2):345–356. doi:10.1016/j.cell.2013.09.048
14. Di Micco R, Krizhanovsky V, Baker D, d’Adda Di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75–95. doi:10.1038/s41580-020-00314-w
15. Chiba K, Lorbeer FK, Shain AH, et al. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science. 2017;357(6358):1416–1420. doi:10.1126/science.aao0535
16. Jeayeng S, Wongkajornsilp A, Slominski AT, Jirawatnotai S, Sampattavanich S, Panich U. Nrf2 in keratinocytes modulates UVB-induced DNA damage and apoptosis in melanocytes through MAPK signaling. Free Radic Biol Med. 2017;108:918–928. doi:10.1016/j.freeradbiomed.2017.05.009
17. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. doi:10.1056/NEJM199711133372003
18. Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth Factor-β Type II receptor/smad signaling. Am J Pathol. 2004;165(3):741–751. doi:10.1016/s0002-9440(10)63337-8
19. Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81(2):807–869. doi:10.1152/physrev.2001.81.2.807
20. Lee HJ, Hwang E, Park B, et al. Methanol extract of bitter melon alleviates UVB-Induced MMPs Expression via MAP kinase and AP-1 signaling in human dermal fibroblasts in vitro. Phytother Res. 2016;30(9):1519–1526. doi:10.1002/ptr.5656
21. Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17(6):868. doi:10.3390/ijms17060868
22. Yokose U, Hachiya A, Sriwiriyanont P, et al. The endogenous protease inhibitor TIMP-1 mediates protection and recovery from cutaneous photodamage. J Invest Dermatol. 2012;132(12):2800–2809. doi:10.1038/jid.2012.204
23. Lebiedzinska M, Duszynski J, Rizzuto R, Pinton P, Wieckowski MR. Age-related changes in levels of p66Shc and serine 36-phosphorylated p66Shc in organs and mouse tissues. Arch Biochem Biophys. 2009;486(1):73–80. doi:10.1016/j.abb.2009.03.007
24. Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi:10.1038/379335a0
25. Deeb D, Gao X, Liu Y, Varma NR, Arbab AS, Gautam SC. Inhibition of telomerase activity by oleanane triterpenoid CDDO-Me in pancreatic cancer cells is ROS-dependent. Molecules. 2013;18(3):3250–3265. doi:10.3390/molecules18033250
26. Xuan SH, Park YM, Ha JH, Jeong YJ, Park SN. The effect of dehydroglyasperin C on UVB–mediated MMPs expression in human HaCaT cells. Pharmacol Rep. 2017;69(6):1224–1231. doi:10.1016/j.pharep.2017.05.012
27. Cui B, Wang Y, Jin J, et al. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP expression, through anti-inflammatory, antioxidant, and antiapoptotic properties, and treats photoaging by upregulating VEGF-B expression. Oxid Med Cell Longev. 2022:6037303. PMID: 35028009; PMCID: PMC8752231. doi:10.1155/2022/6037303
28. Shen G, Xu C, Hu R, et al. Comparison of (-)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (-/-) mice. Pharm Res. 2005;22(11):1805–1820. doi:10.1007/s11095-005-7546-8
29. Li Y, Feng YF, Liu XT, et al. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. 2021;38:101771. doi:10.1016/j.redox.2020.101771
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.