Back to Journals » Clinical Interventions in Aging » Volume 19
Relationship Between Hypertension and Hearing Loss: Analysis of the Related Factors
Received 10 January 2024
Accepted for publication 3 May 2024
Published 17 May 2024 Volume 2024:19 Pages 845—856
DOI https://doi.org/10.2147/CIA.S458869
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Yinjing Hou,1 Bo Liu2
1Department of Geriatrics, Beijing Tongren Hospital, Capital Medical University, Beijing, 100730, People’s Republic of China; 2Department of Otolaryngology Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing Institute of Otolaryngology, Key Laboratory of Otolaryngology Head and Neck Surgery (Capital Medical University), Ministry of Education, Beijing, 100730, People’s Republic of China
Correspondence: Bo Liu, Department of Otolaryngology Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing Institute of Otolaryngology, Key Laboratory of Otolaryngology Head and Neck Surgery (Capital Medical University), Ministry of Education, Beijing, 100730, People’s Republic of China, Tel +13601186097, Email [email protected]
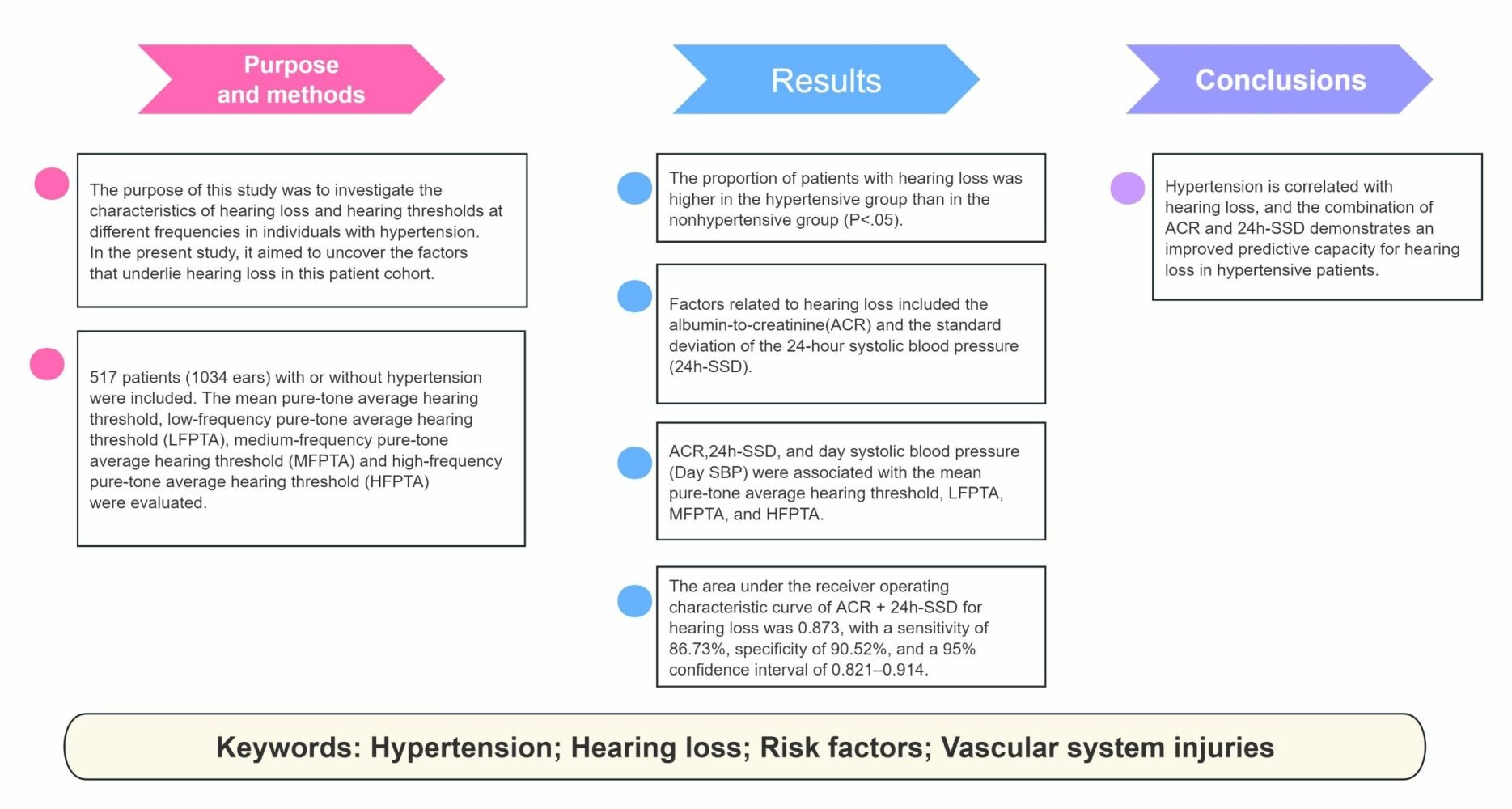
Purpose: The impact of hypertension extends to hearing loss, aging, and mental Health. The purpose of this study was to investigate the characteristics of hearing loss and hearing thresholds at different frequencies in individuals with hypertension. Through a comprehensive analysis, in the present study, it aimed to uncover the contributing factors that underlie hearing loss in this patient cohort, shedding light on the complex relationship between hypertension and auditory impairment.
Patients and Methods: This was a single-center population-based observational study, and clinical, biological, and hospital data were collected from the inpatient ward. In the present study, 517 patients (1034 ears) with or without hypertension were included, and the proportion of patients with hearing loss, mean pure-tone average hearing threshold, low-frequency pure-tone average hearing threshold (LFPTA), medium-frequency pure-tone average hearing threshold (MFPTA) and high-frequency pure-tone average hearing threshold (HFPTA) were evaluated. Risk factors related to hearing loss and hearing threshold were also estimated at different frequencies.
Results: The proportion of patients with hearing loss was higher in the hypertensive group than in the nonhypertensive group (P< 0.05). After including risk factors for cardiovascular disease that can have an impact on the parameters of hearing and ambulatory blood pressure in the regression model, factors related to hearing loss included the albumin-to-creatinine ratio (ACR) and the standard deviation of the 24-hour systolic blood pressure (24h-SSD). ACR, 24h-SSD, and day systolic blood pressure (Day SBP) were associated with the mean pure-tone average hearing threshold, LFPTA, MFPTA, and HFPTA. The area under the receiver operating characteristic curve of ACR + 24h-SSD for hearing loss was 0.873, with a sensitivity of 86.73%, specificity of 90.52%, and a 95% confidence interval of 0.821– 0.914.
Conclusion: Hypertension is correlated with hearing loss, and the combination of ACR and 24h-SSD demonstrates an improved predictive capacity for hearing loss in hypertensive patients.
Keywords: hypertension, hearing loss, risk factors, vascular system injuries
Graphical Abstract:
Introduction
Hypertension is a cardiovascular syndrome characterized by the primary clinical manifestation of elevated systemic arterial pressure, often accompanied by other cardiovascular risk factors that can cause structural and functional damage to vital organs, such as the heart, brain, kidney, and fundus. Hypertension contributes significantly to premature death from cardiovascular disease, due to its high rates of morbidity and mortality. Hearing loss is also prevalent and detrimental, profoundly affecting the daily life and social participation of older adults, and associated with depression, dementia, and even mortality. Although there may be an association between hypertension and hearing loss, current research findings are inconclusive.
Several studies have revealed that hypertension affects the local blood supply to the cochlea, resulting in hearing loss and damage to high-, mid-, and low-frequency hearing thresholds.1–4 The inner ear receives blood from the labyrinthine artery, which is the terminal branch of the vertebrobasilar artery. Consequently, the inner ear is susceptible to vascular diseases that cause disturbances in the local circulation, particularly in the cochlea. This disturbance leads to reduced oxygen and nutrient supply, ultimately affecting hearing. Hypertension is a primary risk factor for peripheral artery disease5 and can induce atherosclerotic changes in small arteries throughout the body. Therefore, hypertension can impair hearing by decreasing blood flow to the cochlea.6,7 Any form of auditory system impairment or alteration of the hearing threshold is considered an early indication of organ damage caused by vascular diseases.8 Therefore, this study aimed to analyze the correlation between clinical indicators of hypertension and hearing in order to gain insights into the impact of blood pressure parameters and vascular system injuries indicators on hearing loss, thereby facilitating the optimization of hypertension management while protecting auditory health.
Materials and Methods
Objectives
In the present study, the objectives were to describe the association between hypertension and hearing loss and to assess factors associated with hearing loss.
Design
This was a population-based, single-center observational study on the characteristics of hearing loss in hypertensive patients, the factors associated with hearing loss, and the hearing threshold at different frequencies in hypertensive patients. Clinical, biological, and hospital data were collected from the inpatient wards.
Setting
The study was conducted on patients in a stable condition who were hospitalized from June 2020 to September 2021. The evaluation of the condition of the patient is under the authority of the team of physicians in charge. This study was conducted in accordance with the Declaration of Helsinki and was approved by the hospital ethics committee. The Ethics Committee of the Beijing Tongren Hospital approved this study (No. TRECKY2021-192). All participants signed informed consent forms.
Participants
Inclusion and Exclusion Criteria
Inclusion criteria were age ≥ 45 years; agreed to participate in the study and met the diagnostic criteria for hypertension according to the 2018 European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) guidelines for the Management of Hypertension [multiple blood pressure measurements in the office (generally three times on different days) ≥ 140/90 mmHg].9 Exclusion criteria were as follows: secondary hypertension; glycosylated hemoglobin > 7.5%; left ventricular ejection fraction ≤ 50%; previous or current diagnosis of myocardial infarction or heart failure, percutaneous coronary intervention, or coronary artery bypass grafting; cerebrovascular diseases (including stroke, transient ischemic attack, revascularization of the carotid or cerebral artery and percutaneous intervention); hyperthyroidism [thyroid stimulating hormone (TSH) < 0.1 mU/L] or hypothyroidism (TSH > 10 mU/L); liver diseases (jaundice hepatitis, cirrhosis and liver failure); chronic kidney disease (stage 4 or above); serious infectious diseases or autoimmune diseases; malignant tumors; mental disorders; history of middle ear disease; history of ototoxic drug poisoning; history of noise-induced deafness and brain trauma; and conductive hearing loss. A total of 517 participants were eligible. According to the diagnostic criteria for hypertension, participants were divided into two groups: the observation group consisted of individuals with hypertension, while the control group comprised individuals without hypertension. See Figure 1 for details on participant inclusion and exclusion.
 |
Figure 1 The procedure for the inclusion and exclusion of subjects. |
Data Collection
Epidemiological Questionnaire
Data were collected from comprehensive case records. The items included demographic information, education, lifestyle (eg, smoking, alcohol consumption, and exercise), duration of hypertension, number of blood pressure medications, and other comorbidities that affect hearing. Alcohol consumption was defined as alcohol consumption more than 12 times in the last 12 months.10 Physical activity was defined as at least 150–300 min of moderate-intensity aerobic activity or 75–150 min of high-intensity aerobic activity per week.11
Albumin-to-Creatinine Ratio
The albumin-to-creatinine ratio (ACR) was determined using urine samples. Urine samples should be collected once, preferably in the morning, to ensure clean midstream samples. Urinary microalbumin was measured by a fluoroimmunoassay, and creatinine was measured by a variation of the Jaffe method on the Beckman CX3. Subsequently, the ratio of urinary microalbumin to creatinine was calculated.12
Blood Pressure Measurement in the Clinic
After resting in a seated position for at least 5 minutes, a corrected Omron HEM-7051 device (Omron HEM-7051, Kyoto, Japan) was used to measure the BP of the participants on their customary arm. Three consecutive blood pressure readings were obtained and averaged to obtain the office-read blood pressure.
Ambulatory Blood Pressure Monitoring
A Vasomedical BIOX, an ambulatory blood pressure monitor, was installed on the patient’s passive arm in the morning to automatically measure and record blood pressure. The monitor was programmed to measure blood pressure at 20-minute intervals between 08:00 and 22:00, and at 60-minute intervals between 22:00 and 08:00 the next morning. The patients wore an ambulatory blood pressure monitor for 24 hours. A record was considered effective if it contained 70% of the programmed readings, the coverage time was greater than 20 hours, and there were at least 20 readings during the day and at least seven readings during the night.13
Pure-Tone Audiometry
A Conera pure-tone audiometer (Madsen, Denmark) was used to perform pure-tone audiometry in a soundproof room. Pure-tone hearing thresholds for both ears were measured at 0.25, 0.5, 1, 2, 4, and 8 kHz. Average hearing thresholds at 0.5, 1, 2, and 4 kHz were considered the mean pure-tone average hearing threshold for both ears. According to the World Health Organization (WHO) classification of degrees of hearing loss, the Clark classification was used.14 Mild hearing loss is classified as 26–40 dB HL, moderate hearing loss as 41–55 dB HL, moderately severe hearing loss as 56–70 dB HL, severe hearing loss as 71–90 dB HL, and profound hearing loss as ≥91 dB HL. The low-frequency pure-tone average hearing threshold (LFPTA) refers to the average of the 0.25 and 0.5 kHz hearing thresholds, the medium-frequency pure-tone average hearing threshold (MFPTA) refers to the average of the 1 and 2 kHz hearing thresholds, and the high-frequency pure-tone average hearing threshold (HFPTA) refers to the average of the 4 and 8 kHz hearing thresholds.
Main Outcome Measures
The proportions of hearing loss were considered, the mean pure-tone average hearing threshold, LFPTA, MFPTA, and HFPTA.
Second, risk factors related to hearing loss, the mean pure-tone average hearing threshold, and LFPTA, MFPTA, and HFPTA were identified.
Statistical Methods
Statistical software (SPSS 22.0) was used for descriptive statistics and difference analysis. The count data were described by frequency distribution and differences were analyzed using the chi-square test. The measurement data are presented as mean ± standard deviation based on the Kolmogorov–Smirnov normality test and ANOVA analyzed by ANOVA. Data that do not conform to the normal distribution are represented as M (Q1, Q2) and were analyzed using the Mann–Whitney U and Kruskal–Wallis tests. All factors were evaluated using a two-sided test, with P < 0.05 considered statistically significant. A multivariate logistic regression analysis was performed with the number of ears as the research object and the hearing loss as the dependent variable. Multifactor linear regression analysis (stepwise method) was performed using mean, low-frequency, medium-frequency, and high-frequency hearing thresholds, as dependent variables and factors related to different hearing thresholds were obtained. The receiver operating characteristic (ROC) curves were constructed to verify the predictive value of factors related to hearing loss.
Results
Patients
A total of 517 eligible participants were enrolled in this study. The average age was 65.00 (56.00, 73.00) years and 303 (58.61%) were men. There were no significant differences in the incidence rates of diabetes, hyperlipidemia, hyperuricemia, family history of cardiovascular disease, coronary atherosclerotic heart disease, or sleep apnea-hypopnea syndrome between the hypertensive and nonhypertensive groups. The left ventricular mass index (LVMI), ACR, cardiovascular ankle index (CAVI), ankle-brachial index (ABI), and increased carotid intima-media thickness (CIMT)/plaque were statistically different between the two groups. The baseline characteristics of the two groups are shown in Table 1.
 |
Table 1 Baseline Characteristics of the Participants |
Detection Parameters
In the hypertensive group, the bilateral mean hearing threshold was 26.25 (18.75, 33.75) dB HL on the left side and 25.00 (18.75, 32.50) dB HL on the right side (P>0.05). In the nonhypertensive group, the bilateral mean hearing threshold was 18.75 (13.44, 26.25) dB HL on the left side, and 19.38 (12.50, 24.06) dB HL on the right side (P>0.05). No significant differences were found in the mean bilateral hearing thresholds of the left and right sides between the groups. The number of ears on both sides was included as an objective of the study. The hypertensive and nonhypertensive groups had 486 and 548 ears, respectively. The hearing thresholds of the two groups are compared in Table 2.
 |
Table 2 Pure-Tone Averages (Air Conduction) of the Participants |
The ambulatory blood pressure parameters between the two groups were compared, as shown in Table 3
 |
Table 3 Ambulatory Blood Pressure Parameters of the Participants |
Analysis of Factors Affecting Hearing Loss and Hearing Threshold at Different Frequencies
The risk factors for cardiovascular disease, the parameters of ambulatory blood pressure, and the subclinical indicators of target organ damage were included in the binary logistic regression model of hearing loss (Table 4 and Figure 2) and the linear regression model of the hearing threshold (Table 5 and Figures 3).
 |
Table 4 Multivariate Binary Logistic Regression Analysis of Hearing Loss |
 |
Table 5 Multivariate Linear Regression Analysis |
Verification of the Predictive Efficacy of ACR and 24h-SSD for Hearing Loss
The area under the ROC curve of ACR for hearing loss was 0.807, sensitivity was 66.33%, specificity was 83.62%, and the 95% confidence interval (CI) was 0.747–0.857. The area under the standard deviation of the 24-hour systolic blood pressure curve (24h-SSD) for hearing loss was 0.775, sensitivity was 74.49%, specificity was 81.90%, and the 95% CI was 0.713–0.829. The area under the curve of ACR+24h-SSD for hearing loss was 0.873, the sensitivity was 86.73%, the specificity was 90.52%, and the 95% CI was 0.821–0.914 (P < 0.001; Figure 4).
Discussion
Hypertension, which can cause structural and functional changes in target organs such as the heart, brain, kidney, and blood vessels, is one of the main causes of cardiovascular disease and premature death. The earliest damage by hypertension is subclinical damage to the target organ, the risk of which is indicated by increased LVMI, structural changes in the carotid arteries, and elevated urinary microalbumin and serum creatinine levels. Hypertensive microangiopathy is a type of subclinical target organ damage indicated by ACR, serum Cr level and LVMI, which are independent predictors of cardiovascular disease and death.15,16 However, the findings of some researchers indicate that hearing loss, particularly in the low-frequency range, can also serve as an indicator of clinical target organ damage resulting from vascular diseases such as hypertension.8
In this study, the hypertensive group exhibited more pronounced subclinical target organ damage and hearing impairment than the nonhypertensive group. Compared with the nonhypertensive group, the hypertensive group showed elevated ACR levels, increased LVMI values, higher bilateral CAVI measurements, decreased bilateral ABI values, and a higher proportion of carotid intima-media thickening/plaque. Furthermore, the hypertension group demonstrated a higher prevalence of hearing loss at the mean pure-tone average hearing threshold and at individual frequencies. Among these indicators, ABI and CAVI serve as markers of atherosclerosis and arterial stiffness, respectively, while ACR and LVMI indicate damage to the microvascular target organ in hypertension. These indicators have a significant clinical predictive value for subclinical target organ damage in hypertension. Therefore, the simultaneous appearance of hearing loss with these indicators may also be associated with early vascular damage caused by hypertension, which is consistent with previous studies.8
In this study, the risk factors for hearing loss in patients with hypertension included ACR and 24h-SSD. Risk factors for the mean pure-tone average hearing threshold, LFPTA, MFPTA, and HFPTA had ACR, 24h-SSD, and daily SBP. The two groups were matched for diabetes and hyperlipidemia to minimize the confounding effects of factors other than hypertension on hearing outcomes. Despite the differences in age between the groups, regression analysis and age adjustment revealed significant associations between ACR, 24h-SSD, day systolic blood pressure (Day SBP), and hearing thresholds.
Although the exact mechanism underlying the influence of hypertension on the hearing threshold remains unclear, this study discovered that injuries to the vascular system can potentially contribute to hearing loss. The findings of this study indicated that both 24h-SSD and Day SBP, which have been shown to affect the hearing threshold, encompassed not only SBP itself but also its variability. This finding suggests that both the level and variability of SBP may affect the hearing threshold, which is consistent with previous studies. For example, a retrospective study conducted in Japan revealed a correlation between increased SBP and higher hearing thresholds at 1 kHz.15 Similarly, a large cross-sectional study conducted in China demonstrated an association between SBP variability and hearing loss.16 SBP is closely associated with vascular system injuries and plays a crucial role in the development of hypertension-induced target organ damage caused by hypertension.17 A meta-analysis involving 16 studies revealed that for every 20 mmHg increase in SBP, there was a corresponding increase of 1.14 m/s in pulse wave velocity, indicating an association between SBP and the progression of atherosclerosis.18
Furthermore, SBP variability is more important in predicting cardiovascular disease risk and all-cause mortality compared to SBP alone.19,20 Similarly, ACR is strongly correlated with arterial stiffness, serves as an indicator of vascular endothelial function, and is associated with an elevated risk of cardiovascular events.21 Therefore, SBP, its variability, and ACR serve as indicators of vascular system injuries. In the present study, it was hypothesized that hypertension-induced vascular system injuries exacerbate local circulatory disorders within the cochlear region, which may contribute to hearing loss due to the absence of collateral circulation within these terminal branches. Therefore, vascular system injuries may play a role in the pathogenesis of hypertension-induced hearing loss. It was discovered that the combination of ACR and 24h-SSD exhibited a discernible predictive effect for hypertension and hearing loss, as demonstrated by the ROC curve. The area under the ROC curve for ACR in patients with both conditions was 0.807, indicating a sensitivity of 66.33% and a specificity of 83.62%. Similarly, the area under the ROC curve for 24h-SSD in these patients was 0.775, with a sensitivity of 74.49% and a specificity of 81.90%. Interestingly, when ACR was combined with 24h-SSD, the area under the ROC curve increased to an impressive value of 0.873, with a sensitivity of 86.73% and a specificity of 90.52%. Consequently, combining these two parameters of vascular system injuries produces higher sensitivity and specificity in diagnosing hypertension with hearing loss than using either parameter individually, indicating that its diagnostic potential for this condition exists to some extent. However, more studies are needed to explore optimal prediction models. Although limited studies have been conducted on the molecular mechanism underlying hypertension-induced hearing loss, several investigations have identified genes associated with hearing loss. Future research can integrate these findings to further elucidate the mechanisms involved in hypertension-induced hearing loss.22,23
This study has several limitations. First, The study design failed to consider the utilization of antihypertensive drugs, thus resulting in a missed opportunity to investigate the impact of such drugs on hearing. In future research, the study design should be improved. In this manner, it could be verified either the hearing loss could be contributed to hypertension or as an effect of antihypertensive drugs. Second, this was a single-center study with a small sample size, and it was not easy to match age and comorbidities between the hypertensive and nonhypertensive groups.Third, due to the limited study time, follow-up could not be performed. These findings need to be verified in future prospective studies.
Conclusions
In conclusion, the findings of this study collectively support a correlation between hypertension and hearing loss, suggesting that vascular damage contributes to hypertension-related hearing loss. In light of these findings, greater attention should be paid to the impact of hypertension on auditory function in clinical practice, with a focus on ensuring blood pressure standards and reducing variability to optimize hearing protection. At the same time, it is recommended that more laboratory studies be conducted in the future to clarify the pathogenesis of hypertension mediated hearing loss.
Abbreviations
24h-SSD, the standard deviation of 24-hour systolic blood pressure; 95% CI, 95% Confidence Interval; ABI, ankle-brachial index; ACR, albumin-to-creatinine ratio; CAVI, cardiovascular ankle index; CIMT, carotid intima-media; Day SBP, day systolic blood pressure; ESC, European Society of Cardiology; ESH, European Society of Hypertension; FPTA, high-frequency pure-tone average hearing threshold; LVMI, Left ventricular mass index; LFPTA, low-frequency pure-tone average hearing threshold; MFPTA, medium-frequency pure-tone; average hearing threshold; TSH, Thyroid stimulating hormone.
Data Sharing Statement
The datasets generated and analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Ethics Approval
The study followed the Declaration of Helsinki and was approved by the hospital ethics committee (Beijing Tongren Hospital, Capital Medical University, ethics number TRECKY2021-192).
Consent to Participate
All the participants signed informed consent forms.
Acknowledgments
We would like to thank Editage (www.editage.cn) for the English language editing. This paper has been uploaded to Authorea as a preprint: https://www.authorea.com/users/593747/articles/628598-relationship-between-hypertension-and-hearing-loss-and-analysis-of-The-related-factors.
Author Contributions
All authors made significant contributions to the work reported, whether in concept and design, implementation, data acquisition, analysis, and interpretation, or all of these areas; participated in the drafting of the article or the critical revision of important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to take responsibility for all aspects of the work.
Funding
This work was supported by the Natural Science Research Foundation of Beijing Tongren Hospital, Capital Medical University [grant number: 2021-YJJ-ZR-013].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kirbac A, Boke B. Effects of primary arterial hypertension on cochlear function. Acta Oto-Laryngol. 2021;141(2):158–162. doi:10.1080/00016489.2020.1856923
2. Hara K, Okada M, Takagi D, et al. Association between hypertension, dyslipidemia, and diabetes and prevalence of hearing impairment in Japan. Hypertens Res. 2020;43(9):963–968. doi:10.1038/s41440-020-0444-y
3. Wattamwar K, Qian ZJ, Otter J, et al. Association of cardiovascular comorbidities with hearing loss in the older old. JAMA Otolaryngol Head Neck Surg. 2018;144(7):623–629. doi:10.1001/jamaoto.2018.0643
4. Tan HE, Lan NSR, Knuiman MW, et al. Associations between cardiovascular disease and its risk factors with hearing loss-A cross-sectional analysis. Clin Otolaryngol. 2018;43(1):1721–1781. doi:10.1111/coa.12936
5. Matsunaga M, Yatsuya H, Iso H, et al. Similarities and differences between coronary heart disease and stroke in the associations with cardiovascular risk factors: the Japan Collaborative Cohort Study. Atherosclerosis. 2017;261:124–130. doi:10.1016/j.atherosclerosis.2017.03.003
6. Savoia C, Battistoni A, Calvez V, Cesario V, Montefusco G, Filippini A. Microvascular alterations in hypertension and vascular aging. Curr Hypertens Rev. 2017;13(1):16–23. doi:10.2174/1573402113666170505115010
7. Umesawa M, Sairenchi T, Haruyama Y, Nagao M, Kobashi G. Association between hypertension and hearing impairment in health check-ups among Japanese workers: a cross-sectional study. BMJ Open. 2019;9(4):e028392. doi:10.1136/bmjopen-2018-028392
8. Friedland DR, Cederberg C, Tarima S. Audiometric pattern as a predictor of cardiovascular status: development of a model for assessment of risk. Laryngoscope. 2009;119(3):473–486. doi:10.1002/lary.20130
9. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi:10.1093/eurheartj/ehy339
10. Cunningham SA, Mosher A, Judd SE, et al. Alcohol consumption and incident stroke among older adults. J Gerontol B Psychol Sci Soc Sci. 2018;73(4):636–648. doi:10.1093/geronb/gbw153
11. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462. doi:10.1136/bjsports-2020-102955
12. Younes N, Cleary PA, Steffes MW, et al. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol. 2010;5(7):1235–1242. doi:10.2215/CJN.07901109
13. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi:10.1161/HYPERTENSIONAHA.120.15026
14. Clark JG. Uses and abuses of hearing loss classification. ASHA. 1981;23(7):493–500.
15. Miyata J, Umesawa M, Yoshioka T, Iso H. Association between high systolic blood pressure and objective hearing impairment among Japanese adults: a facility-based retrospective cohort study. Hypertens Res. 2022;45(1):155–161. doi:10.1038/s41440-021-00737-8
16. Bao M, Song Y, Cai J, Wu S, Yang X. Blood pressure variability is associated with hearing and hearing loss: a population-based study in males. Int J Hypertens. 2019;2019:9891025. doi:10.1155/2019/9891025
17. Oparil S, Acelajado MC, Bakris GL, et al. Hypertension. Nat Rev Dis Primers. 2018;4:18014. doi:10.1038/nrdp.2018.14
18. Wilson J, Webb AJS. Systolic blood pressure and longitudinal progression of arterial stiffness: a quantitative meta-analysis. J Am Heart Assoc. 2020;9(17):e017804. doi:10.1161/JAHA.120.017804
19. Wang J, Shi X, Ma C, et al. Visit-to-visit blood pressure variability is a risk factor for all-cause mortality and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2017;35(1):10–17. doi:10.1097/HJH.0000000000001159
20. Clark DR, Nicholls SJ, St John J, et al. Visit-to-visit blood pressure variability, coronary atheroma progression, and clinical outcomes. JAMA Cardiol. 2019;4(5):437–443. doi:10.1001/jamacardio.2019.0751
21. Liu S, Niu J, Wu S, et al. Urinary albumin-to-creatinine ratio levels are associated with subclinical atherosclerosis and predict CVD events and all-cause deaths: a prospective analysis. BMJ Open. 2021;11(3):e040890. doi:10.1136/bmjopen-2020-040890
22. Doll J, Vona B, Schnapp L, et al. Genetic Spectrum of Syndromic and Non-Syndromic Hearing Loss in Pakistani Families. Genes. 2020;11(11):1329. doi:10.3390/genes11111329
23. Buonfiglio P, Bruque CD, Luce L, et al. GJB2 and GJB6 Genetic Variant Curation in an Argentinean Non-Syndromic Hearing-Impaired Cohort. Genes. 2020;11(10):1233. doi:10.3390/genes11101233
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



