Back to Journals » Clinical Interventions in Aging » Volume 9
Visual outcome of intravitreal ranibizumab for exudative age-related macular degeneration: timing and prognosis
Authors Canan H , Sizmaz S, Altan-Yaycıoğlu R, Sarıtürk, Yilmaz G
Received 1 November 2013
Accepted for publication 2 December 2013
Published 10 January 2014 Volume 2014:9 Pages 141—145
DOI https://doi.org/10.2147/CIA.S56863
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Handan Canan,1 Selçuk Sizmaz,2 Rana Altan-Yaycioğlu,1 Çağla Saritürk,3 Gürsel Yilmaz4
1Department of Ophthalmology, Adana Teaching and Medical Research Center, Baskent University School of Medicine, 2Department of Ophthalmology, Çukurova University School of Medicine, 3Department of Biostatistics, Adana Teaching and Medical Research Center, Baskent University School of Medicine, 4Department of Ophthalmology, Baskent University School of Medicine, Ankara, Turkey
Purpose: To describe 1-year clinical results of intravitreal ranibizumab treatment in patients with choroidal neovascularization secondary to exudative age-related macular degeneration (AMD) and to evaluate whether early treatment is a predictive value for prognosis of the disease.
Materials and methods: Clinical records were retrospectively reviewed of 104 eyes that underwent intravitreal ranibizumab therapy for exudative AMD. Patients were divided into two groups according to their symptom duration: group 1, <1 month; and group 2, 1–3 months. After three monthly injections, patients were examined monthly, and subsequent injections were performed as needed.
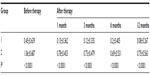
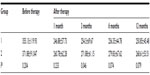
Results: There were 43 female (48.9%) and 45 males (51.1%). The follow-up time was 13.7±1.9 (12–19) months. The mean logarithm of minimum angle of resolution best-corrected visual acuity (BCVA) improved significantly, from 0.45±0.639 at baseline to 0.08±0.267 at 12 months in group 1, and from 1.06±0.687 at baseline to 0.75±0.563 at 12 months in group 2. The increase in BCVA was statistically significant in group 1 (P=0.009). The mean central retinal thickness (CRT) decreased significantly, from 355.13±119.93 µm at baseline to 250.85±45.48 µm at 12 months in group 1, and from 371.88±91.047 µm at baseline to 268.61±53.51 µm at 12 months in group 2. The decrease in CRT was statistically significant in group 1 (P=0.001).
Conclusion: Intravitreal ranibizumab therapy was effective in significantly increasing mean BVCA and reducing CRT. Shorter duration of AMD, as measured by the subjective duration of visual symptoms, is associated with better visual outcome after treatment.
Keywords: age-related macular degeneration (AMD), optical coherence tomography (OCT), ranibizumab, visual acuity
Introduction
Age-related macular degeneration (AMD) is the leading cause of vision loss in the world. Choroidal neovascularization (CNV) plays the main role in visual deterioration in exudative AMD.1–3 CNV originating from choriocapillaris and invading the retina is the result of ongoing inflammation that is mainly stimulated by vascular endothelial growth factor (VEGF).4–6 Inhibition of VEGF by anti-VEGF agents can improve prognosis in exudative AMD by suppressing angiogenesis and decreasing vascular permeability.7,8 Major clinical trials have reported the superior efficacy of ranibizumab, which is a US Food and Drug Administration-approved anti-VEGF agent (Lucentis; Genentech, San Francisco, CA, USA).9,10 Thus, intravitreal injection of ranibizumab has become the standard therapy for AMD worldwide.11 However, there are still controversial issues on the efficiency of ranibizumab and visual outcome. The delay between the onset of symptoms and the timing of the therapy is reported to be in negative correlation with the outcome.6,12 In AMD, CNV was found to enlarge at an average of 10 (1–24) μm per day.13 Although the predictive value of this rate is small, it is important to stress the timing of treatment initiation. The purpose of the current study was to report the 1-year clinical results of intravitreal ranibizumab treatment in patients with exudative AMD, and to evaluate whether early treatment is of predictive value for prognosis of the disease.
Materials and methods
Medical reports of patients who underwent intravitreal injections of ranibizumab for new-onset exudative AMD between March 2010 and January 2013 at Başkent University School of Medicine Department of Ophthalmology were reviewed retrospectively. This study was approved by the Baskent University Institutional Review Board and Ethics Committee. The following parameters were achieved from patient’s chart at baseline and after 12 months: all patients underwent full ophthalmic examination, which included best-corrected visual acuity (BCVA), intraocular pressure, slit-lamp biomicroscopy, and dilated fundus examination. Central retinal thickness (CRT) measurements were done by spectral domain optical coherence tomography (OCT) (RTVue; Optovue, Fremont, CA, USA). Fluorescein angiography was performed at initial diagnosis. In the charts, BCVA was assessed using Snellen visual acuity and it was converted to a logarithm of the minimum angle of resolution (logMAR) value for statistical analysis. BCVA assessments were done by the same clinic staff who recorded the data. All patients were treated with three consecutive monthly intravitreal injections of ranibizumab and followed up for at least 12 months. After three monthly injections, patients were examined monthly, and subsequent injections were performed as needed (pro re nata) for any recurrence. Recurrence was defined as decrease of at least one line in visual acuity, or presence of new macular hemorrhage on CNV and the presence of intraretinal and/or subretinal fluid, and an increase of 50 μm or over in central retinal thickness in OCT scans. Written informed consent was obtained from all patients before administration of the intravitreal ranibizumab. After the eye had been prepared using 5% povidone–iodine, 0.5 mg of ranibizumab was injected via the pars plana. The duration of the disease was quantified according to the patients’ history from the very beginning of visual symptoms (visual distortion, changes in vision acuity, or central blurring) to initial presentation. Patients were divided into two groups: group 1 consisted of patients with visual symptoms for less than 1 month, and patients who had visual symptoms for 1–3 months were placed in group 2. Included patients had symptoms for a maximum of 3 months, underwent at least three injections, and completed 1-year follow-up. The exclusion criteria were any previous treatment for CNV and CNV that was not associated with AMD.
Statistical analysis
Statistical analysis was performed using the statistical package SPSS version 17.0 (IBM, Armonk, NY, USA). For each continuous variable, normality was checked by Kolmogorov–Smirnov and Shapiro–Wilk tests and by histograms. Comparisons between groups were applied using the Mann–Whitney U test for data not normally distributed. Pre- and postinjection changes were analyzed with Wilcoxon and repeated-measures analyses (Greenhouse–Geisser). Values of P<0.05 were considered statistically significant.
Results
A total of 104 eyes of 88 patients were involved in the study; there were 40 eyes in group 1 (16.2±4.8 days, 7–30 days), and 64 eyes in group 2 (53.1±14.2 days, 35–90 days). The mean age of the patients was 68.5±9.6 (50–87) years. There were 43 female (48.9%) and 45 males (51.1%). The follow-up time was 13.7±1.9 (12–19) months. At baseline, the mean logMAR BCVA was 0.45±0.639 (0–3) in group 1 and 1.06±0.687(0–3) in group 2. The mean logMAR BCVA values per group after treatment are shown in Table 1. The increase in VA was statistically significant for the first, third, sixth, ninth, and twelfth months in group 1 and group 2 compared to baseline (P<0.0001). The mean logMAR BCVA improved significantly, from 0.45±0.639 at baseline to 0.08±0.267 at 12 months in group 1, and from 1.06±0.687 at baseline to 0.75±0.563 at 12 months in group 2. The increase in BCVA was statistically significant in group 1 (P=0.009). At the final follow-up, BCVA was increased in 56.7% of the eyes and decreased in 14.5% of the eyes. In 30 (28.8%) eyes, visual acuity remained unchanged at 12 months. Baseline mean CRT was 355.13±119.93 (200–775) μm in group 1 and 371.88±91.047 (234–714) μm in group 2. Mean CRT values per group after treatment are shown in Table 2. The decrease in CRT was statistically significant for the third month between group 1 and group 2 compared to baseline (P=0.046). Mean CRT decreased significantly, from 355.13±119.93 μm at baseline to 250.85±45.48 μm at 12 months in group 1, and from 371.88±91.047 μm at baseline to 268.61±53.51 μm at 12 months in group 2. The decrease in CRT was statistically significant in group 1 (P=0.001). A graphical representation of CRT and BCVA over time is shown in Figures 1 and 2. The mean number of intravitreal ranibizumab injections applied in the 12-month period was 4.32 (range 3–9). The mean number of injections was 4.57±1.4 (3–9) in group 1 and 4.17±0.9 (3–6) in group 2. There was no significant difference between the two groups (P=0.092). No inflammation, infection, ocular toxicity signs, or systemic side effects were seen.
  | Figure 2 Change in CRT before and after ranibizumab therapy. |
Discussion
Several clinical key trials, such as MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular AMD), ANCHOR (Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD), and SAILOR (A Study to Evaluate Ranibizumab in Subjects with Choroidal Neovascularization [CNV] Secondary to Age-Related Macular Degeneration) have demonstrated the efficacy and safety of ranibizumab injections for the entire spectrum of CNV subtypes.9–11,14,15 In the PrONTO (Prospective OCT Study with Lucentis for Neovascular AMD) trial, after the three consecutive monthly intravitreal injections, further injections were administered according to changes in BCVA, OCT, and ophthalmoscopic macula findings.16,17 These reinjection criteria were also used in our study. Results of the PrONTO study (mean improvement in BCVA of 9.2 letters, mean 5.6 injections) were comparable with MARINA or ANCHOR, which assumes stabilization and improvement of visual acuity with a decreased number of injections.16 In our study, a mean of 4.32 (3–9) injections were performed during a 12-month period to achieve recovery of BCVA and CRT parameters. In the PrONTO study, the mean CRT was 394 μm at baseline and 216 μm at 12 months.16 In our study, mean CRT declined from 365.43±102.87 μm to 261.78±51.09 μm at 12 months. Reduction in CRT was statistically significant in group 1 (P=0.001). A shorter duration of visual symptoms (group 1) was correlated with a higher initial and final BCVA (P=0.009). Rauch et al reported that CNV with shorter disease duration yielded better visual outcome when compared with eyes with longer duration after two injections per eye at the 6-month follow-up.6 They particularly reported favorable results in patients with a duration of less than 1 month. Oliver-Fernandez et al reported that 44% of patients lost vision and 15% lost more than three lines of visual acuity in 28 days.12 Muether et al also stressed the negative effect of delay in treatment on visual outcome in two recent reports.18,19 Analysis of our results demonstrates that intravitreal ranibizumab treatment for exudative AMD clearly preserved and improved mean visual acuity in our patients. Shorter duration of AMD, as measured by the subjective duration of visual symptoms, was associated with a better visual outcome after treatment. The retrospective design and the small sample size are perhaps limitations of our study. The duration of the disease was based on the subjective history given by the patients. This is also considered as a shortcoming. However, there is no way to assess objective duration. Our study was designed to retrospectively analyze 1-year clinical results of intravitreal ranibizumab injections in patients with exudative AMD and to stress that early diagnosis and prompt therapy are associated with favorable outcomes. Currently, intravitreal ranibizumab is the gold-standard therapy for exudative AMD. Better baseline visual acuity and early treatment is essential. Patients must be informed about self-awareness of visual symptoms. Prospective randomized studies with larger sample sizes would reveal more predictive results.
Disclosure
The authors report no conflicts of interest in this work.
References
Emerson MV, Lauer AK. Current and emerging therapies for the treatment of age-related macular degeneration. Clin Ophthalmol. 2008;2:377–388. | |
Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. | |
Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1220–1231. | |
Kvanta A, Algvere PV, Berglin L, Sereqard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–1934. | |
Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81:154–162. | |
Rauch R, Weingessel B, Maca SM, Vecsei-Marlovits PV. Time to first treatment: the significance of early treatment of exudative age-related macular degeneration. Retina. 2012;32:1260–1264. | |
Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144:850–857. | |
Cleary CA, Jungkim S, Ravikumar K, Kelliher C, Acheson RW, Hickey-Dwyer M. Intravitreal bevacizumab in the treatment of neovascular age-related macular degeneration, 6 and 9 month results. Eye (Lond). 2008;22:82–86. | |
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. | |
Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. | |
Mitchell P, Korobelnik JF, Lanzetta P, et al. Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol. 2010;94:2–13. | |
Oliver-Fernandez A, Bakal J, Segal S, Shah GK, Dugar A, Sharma S. Progression of visual loss and time between initial assessment and treatment of wet age-related macular degeneration. Can J Ophthalmol. 2005;40:313–319. | |
Vander JF, Morgan CM, Schatz H. Growth rate of subretinal neovascularization in age-related macular degeneration. Ophthalmology. 1989;96:1422–1426. | |
Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116:1731–1739. | |
Fong AH, Lai TY. Long-term effectiveness of ranibizumab for age-related macular degeneration and diabetic macular edema. Clin Interv Aging. 2013;8:467–483. | |
Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. | |
Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148:43–58. | |
Muether PS, Hoerster R, Hermann MM, Kirchhof B, Fauser S. Long-term effects of ranibizumab treatment delay in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2013;251:453–458. | |
Muether PS, Hermann MM, Koch K, Fauser S. Delay between medical indication to anti-VEGF treatment in age-related macular degeneration can result in a loss of visual acuity. Graefes Arch Clin Exp Ophthalmol. 2011;249:633–637. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.