Back to Journals » Clinical and Experimental Gastroenterology » Volume 8
Value of portal venous system radiological indices in predicting esophageal varices
Authors Gaduputi V , Patel H , Sakam S, Neshangi S, Ahmed R, Lombino M, Chilimuri S
Received 29 October 2014
Accepted for publication 10 January 2015
Published 9 February 2015 Volume 2015:8 Pages 89—93
DOI https://doi.org/10.2147/CEG.S76579
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Andreas M. Kaiser
Vinaya Gaduputi,1 Harish Patel,1 Sailaja Sakam,1 Srivani Neshangi,1 Rafeeq Ahmed,1 Michael Lombino,2 Sridhar Chilimuri1
1Department of Medicine, 2Department of Radiology, Bronx Lebanon Hospital Center New York, NY, USA
Introduction: Portal hypertension results from increased resistance to portal blood flow and has the potential complications of variceal bleeding and ascites. The splenoportal veins increase in caliber with worsening portal hypertension, and partially decompress by opening a shunt with systemic circulation, ie, a varix. In the event of portosystemic shunting, there is a differential decompression across the portal vein and splenic vein (portal vein > splenic vein), with a resultant decrease in the ratio of portal vein diameter to that of splenic vein. Portal vein to splenic vein diameter ratio and gradient could be valuable tools in predicting the presence of portosystemic shunting.
Methods: We retrospectively reviewed patients with cirrhosis who underwent esophagogastroduodenoscopy (EGD) for variceal screening and had a computerized tomogram (CT) of the abdomen within 6 months of the index endoscopic study, between January 2009 and December 2013. Patients on nonselective beta blockers, patients with presinusoidal portal hypertension (portal vein thrombosis or extrinsic compression), and patients who had undergone portosystemic shunting procedures (transjugular intrahepatic portosystemic shunt [TIPS]) or balloon-occluded retrograde transvenous obliteration (BRTO) were excluded from the study. Splenic and portal vein diameters were measured (in mm) just proximal and distal to the splenomesenteric venous confluence, respectively.
Results: A total of 164 patients were included in the study; of these, 60% (n=98) were male and 40% (n=66) were female. The mean age of the study population was 58.7 years. A total of 126 patients (77%) had varices, while 38 patients (33%) did not. The mean Model for End-Stage Liver Disease (MELD) score was 5.9 for those who had varices as compared with 7.03 for those who did not. The mean of ratios of portal vein to splenic vein diameters in patients with varices was 1.27 (±0.2), while it was 1.5 (±0.23) in those without varices. This difference was statistically significant (P<0.001). The mean of the gradients between the portal vein and splenic vein diameters was 2.7 (±2) mm for patients with varices as compared with 5 (±1.8) mm in those without varices. This difference was also statistically different (P<0.001). These correlations were statistically significant even after controlling for age, sex, and MELD. These radiological indices also had statistically significant correlations with the presence of gastric varices (P=0.018 for the ratio and P=0.01 for the gradient). A discriminant function analysis was performed that generated the equation: D = 2.68 (ratio of portal vein to splenic vein diameters) + 0.187 (gradient of portal vein to splenic vein diameters, in mm) - 4.152. This equation had a very high sensitivity, of 95%, but low specificity, of 26.3%, in predicting the presence of esophageal varices.
Conclusion: Both venous diameter ratio (portal vein size/splenic vein size) and venous diameter gradient in mm (portal vein size – splenic vein size) calculated from CTs of the abdomen were good predictors of presence of esophageal varices. These parameters might be useful in stratifying patients at risk of developing esophageal varices who are poor candidates for endoscopic evaluation.
Keywords: portal vein diameter, splenic vein diameter, portal hypertension, portal vein to splenic vein ratio, portosplenic venous size gradient
Introduction
Cirrhosis is progressive hepatic fibrosis characterized by distortion of the hepatic architecture. It has been identified as one of the leading causes of mortality, with close to 50,000 deaths attributed to it per year in the United States.1 The major morbidity from cirrhosis is due to portal hypertension, with formation of venous collaterals and marked circulatory as well as vascular abnormalities. Portal hypertension is a manifestation of increased resistance to portal blood flow, resulting most commonly from structural and dynamic changes within a fibrotic liver.2 A majority of patients with cirrhosis have elevated portal pressure gradient, with more than one-third developing esophageal varices.3 The rate of development of new varices in patients with cirrhosis is about 8% per year.4 Bleeding from varices accounts for a significant proportion of all deaths related to cirrhosis.5 The mortality rate of variceal bleed approaches 30% with an additional one-third of patients dying within a year.6 It has therefore been recommended that all patients with cirrhosis undergo endoscopic screening for varices.7
Even though an elevated hepatic-portal vein pressure gradient of >10 mm of Hg is the single most accurate predictor for development of varices,8 its measurement is often hampered by lack of technical expertise and is fraught with complications, such as intraperitoneal bleeding. Attempts have been made to diagnose portal hypertension with noninvasive radiological tests, such as ultrasound. Several parameters, including portal vein size, flow reversal, and thrombosis have been looked into but were found to be lacking sensitivity.9 However, no studies have been done previously to demonstrate the sensitivity of portal vein to splenic vein diameter ratio and gradients, as measured with computerized tomography (CT), in predicting esophageal varices. In this retrospective study, we looked at the value of measuring these radiological indices in patients with cirrhosis undergoing esophagogastroduodenoscopy (EGD) for variceal screening.
Methods and materials
Patients
This retrospective study was performed according to the Declaration of Helsinki. The Institution Review Board (IRB) at Bronx Lebanon Hospital Center approved the protocol. The period of study was 5 years, from 2009 to 2013. The data was collected from the electronic medical records of patients and tabulated in Microsoft Excel® (Microsoft Corp, Redmond, WA, USA). All patients with cirrhosis who underwent EGD for variceal screening and also had a contrast enhanced CT of abdomen within 6 months of the index endoscopic procedure were included in the study. All the endoscopies were performed at a single center by experienced gastroenterologists using Olympus GIF-160 gastrointestinal videoscopes (Olympus Corp, Tokyo, Japan). Patients on nonselective beta blockers, patients with presinusoidal portal hypertension (portal vein thrombosis or extrinsic compression), and patients with transjugular intrahepatic portosystemic shunt (TIPS) or balloon-occluded retrograde transvenous obliteration (BRTO) were excluded from the study.
The endoscopic reports of all the patients included within the study were manually reviewed. Patients were allocated to either of the two groups: group 1 with esophageal varices and group 2 without esophageal varices. The grading of varices was done using the Beppu Classification.10 We collected the baseline demographic data for both groups, including age, sex, and race. We calculated the Model for End Stage Liver Disease (MELD) scores for both groups at the time of the procedure.
Evaluation of results
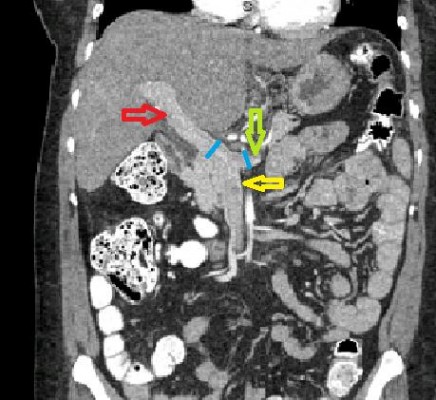
We measured the sizes of portal vein and splenic veins on CTs of the abdomen (in mm). All the measurements were taken within 5 mm of the splenomesenteric confluence and in the coronal plane (as shown in Figure 1). The ratio and difference of these measurements were computed. The means of these computed values in the varices group were then compared with those in the group without the varices.
Statistical analysis
Continuous variables were stated as mean (± standard deviation) and categorical variables as variceal presence or absence. Fisher’s exact test was used for comparing two categorical variables. Unpaired t-test was used for comparison of means. The categorical and continuous variables were compared using analysis of variance (ANOVA). Efficiency of the computed parameters in predicting the studied outcome was calculated using discriminant function analysis. A P-value <0.05 was considered statistically significant.
Results
There were a total of 164 unique patients with cirrhosis who underwent EGD for variceal screening and contrast enhanced CT of abdomen within the study period of January 2009 to December 2013. Out of the study population, 126 patients (67%) had varices while 38 patients (33%) did not. The baseline characteristics of both study groups are shown in Table 1. We found that all the baseline characteristics were similar among these two groups barring mean age (significantly higher in the varices group [P=0.0118]) and MELD scores (significantly higher in the nonvarices group [P=0.0116]). The distribution of etiologies of liver cirrhosis was not significantly different amongst the two groups (P=0.7261).
The mean of ratio of portal vein to splenic vein diameters in patients with varices was 1.27 (±0.2) as compared with 1.5 (±0.23) in those without (Figure 2). The difference was statistically significant, with a P-value of <0.001.
The mean of the gradients between the portal vein and splenic vein diameters was 2.7 (±2) mm for patients with varices as compared with 5 (±1.8) mm for those without varices (Figure 3). This was also significantly different between the two groups, with a P-value of <0.001.
These correlations were maintained even after controlling for age, MELD score, sex, or etiology of cirrhosis.
Neither the ratio of portal vein to splenic vein diameters (P=0.76) nor the gradient between the portal vein and splenic vein (P=0.90) correlated with the grade of esophageal varices. However, there was a significant correlation between the mean ratio of portal vein to splenic vein diameters, and the presence of gastric varices (P=0.018). The same was true for the correlation between the mean gradient between the portal vein and splenic vein, and the presence of gastric varices (P=0.01).
Univariate analysis showed that the mean ratios of the splenic vein to portal vein diameters was significant, and so was the difference between them, in predicting the presence of varices. Hence, to further evaluate the efficiency of these parameters in predicting varices, a discriminant function analysis model was computed. As per the analysis, the log determinants were similar – Box’s M (0.0096) indicated that assumption of equality of the covariance matrices was violated (>0.001). The canonical discriminant function value was 0.407, and the utility of this model to predict the outcome of varices was significant (Wilk’s lambda with significance of <0.001). The weighted mean of the centroids was 0.0282. The sensitivity of predicting the varices with this model was 95%; however, the specificity was low, at only 26.3%. The positive predictive value of the model was calculated to be 81.08%, whereas the negative predictive value was 62.5%. The distribution of the discriminant score can be seen in Figure 4. The following was generated from the canonical discriminant function coefficients.

where D is the discriminant function coefficient.
  | Figure 4 Distribution of the discriminant score. |
We also looked at correlation between the portal venous system radiological indices and validated noninvasive esophageal varices assessment tools, such as platelet count/spleen volume ratio. There was no correlation between the portal vein splenic vein diameter ratio and platelet count/spleen volume ratio (P-value= 0.499). The same was true with the correlation between portal vein splenic vein diameter gradient and platelet count/spleen volume ratio (P-value = 0.2) (Figure 5).
Discussion
The hemodynamics of portal venous flow is yet to be fully elucidated. Increased resistance to portal venous flow, due to hepatic fibrosis with subsequent collateral vessel formation and diffuse vasodilatation, contributes to the progression of portal hypertension.11 The typical sonographic changes in portal hypertension include biphasic or reversal of flow in the portal vein or recanalization of the paraumbilical vein (enhancement in a CT study). Presence of venous collaterals also point towards portal hypertension, but these findings are often missed in presence of obesity and bowel gas.12 Consistent with the hydraulic analogy to Ohm’s law (Pressure = Resistance × Flow), we hypothesized that with a fixed resistance from a fibrotic liver, increased flow, with collateral formation, is seen in advanced portal hypertension. The splenoportal veins increase in caliber with worsening portal hypertension, and partially decompresses by opening a shunt with systemic circulation, ie, a varix. In the event of portosystemic shunting; there is a differential decompression across the portal vein and splenic vein (portal vein > splenic vein), with a resultant decrease in the ratio of portal vein diameter to that of splenic vein. The portal vein to splenic vein diameter ratio and gradient could be valuable tools in predicting the presence of portosystemic shunting. We found that both these radiological indices correlated well with presence of esophageal varices.
Our study had multiple disadvantages that are inherent to a retrospective study. Even though we took only patients with liver cirrhosis undergoing EGD for variceal screening who did not have any of the factors that could directly influence the portal or splenic vein pressures (as identified by medication reconciliation or imaging findings), we allowed a maximum period of up to a 6 months between the endoscopic study and CT scan, which could have skewed our findings. Though CT imaging does not suffer from interoperator variability, unlike ultrasonogram, there could be interobserver variability in measuring the portal venous system diameters. Even though the sample size was small, we did achieve high sensitivity of 95% with our prediction model.
In conclusion, we observed statistically significant correlation between portal to splenic vein ratio and gradients to the presence of esophageal varices. This could be especially useful in patients with end-stage liver disease who are high risk for undergoing diagnostic endoscopy under intravenous sedation. However, we recommend prospective studies with larger sample sizes to clearly elucidate the value of radiological indices in predicting the presence of esophageal varices.
Author contributions
All Authors have made contributions to the article and have reviewed it before submission.
Disclosure
The authors report no conflicts of interest in this work.
References
US Burden of Disease Collaborators. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. | |
García-Pagán JC, Gracia-Sancho J, Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57(2):458–461. | |
Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7(2):141–155. | |
Merli M, Nicolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38(3):266–272. | |
North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319(15):983–989. | |
Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80(4):800–809. | |
Grace ND, Groszmann RJ, Garcia-Tsao G, et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology. 1998;28(3):868–880. | |
Groszmann RJ, Garcia-Tsao G, Bosch J, et al; Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353(21):2254–2261. | |
Berzigotti A, Piscaglia F; EFSUMB Education and Professional Standards Committee. Ultrasound in portal hypertension – part 2 – and EFSUMB recommendations for the performance and reporting of ultrasound examinations in portal hypertension. Ultraschall Med. 2012;33(1):8–32; quiz 30. | |
Beppu K, Inokuchi K, Koyanagi N, et al. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27(4):213–218. | |
Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis – current status and future directions. J Hepatol. 2014;61(4):912–924. | |
Kok T, van der Jagt EJ, Haagsma EB, Bijleveld CM, Jansen PL, Boeve WJ. The value of Doppler ultrasound in cirrhosis and portal hypertension. Scand J Gastroenterol Suppl. 1999;230:S82–S88. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.