Back to Journals » Medical Devices: Evidence and Research » Volume 8
Validation of the BPLab® 24-hour blood pressure monitoring system in a pediatric population according to the 1993 British Hypertension Society protocol
Authors Ledyaev M, Stepanova O, Ledyaeva A
Received 2 December 2014
Accepted for publication 31 December 2014
Published 2 February 2015 Volume 2015:8 Pages 115—118
DOI https://doi.org/10.2147/MDER.S78515
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Mikhail Y Ledyaev, Olga V Stepanova, Anastasia M Ledyaeva
Department of Pediatric Disease, Volgograd State Medical University, Volgograd, Russian Federation
Background: Automatic 24-hour ambulatory blood pressure (BP) monitoring (ABPM) is a basic procedure performed in adults with arterial hypertension, but ABPM monitors have become widely used in pediatric practice only recently. The main problem is the lack of common normative data sets for ABPM in children and the small number of appropriate monitors that can be used for analysis of the 24-hour BP profile in this age group. The aim of this study was to validate the BPLab® ABPM monitor according to the 1993 British Hypertension Society (BHS-93) protocol, as well as to work out solutions regarding the feasibility of this device in pediatric practice.
Methods: Our study included 30 children of both sexes and aged 5–15 years, ie, “older” children according to the BHS-93 protocol. Before starting the study, we obtained ethical approval from the regional scientific ethics committee. All participants and their parents signed their written consent for participation in the study. The data were simultaneously obtained by three experts, who had completed a noninvasive BP measurement training course. BP values were measured using the Korotkoff auscultatory method (Phase I for systolic BP and Phase V for diastolic BP). Discrepancies in the systolic and diastolic BP measurements (n=180; 90 for each expert) were analyzed according to the criteria specified in the BHS-93 protocol.
Results: The device was graded “A” for both systolic BP and diastolic BP according to the criteria of the BHS-93 protocol.
Conclusion: The BPLab ABPM device may be recommended for extensive pediatric use.
Keywords: ambulatory blood pressure monitoring, children, device, validation
Introduction
Automatic noninvasive blood pressure (BP) measurement is widely used in adults for self-monitoring and for clinical evaluation of the circadian BP profile. Ambulatory BP monitoring (ABPM) is added to the standard plan for the diagnosis and treatment of adults with arterial hypertension according to the European Society of Hypertension and European Society of Cardiology guidelines. In recent years, ABPM has been more widely used in the pediatric population.1–4 Use of ABPM in all children and adolescents with hypertension can be even more important than in adults according to the recommendations of the European Society of Hypertension.4 However, the lack of common normative data for ABPM in children and the small number of appropriate monitors recommended for evaluation of the circadian BP profile prevent that method from being widely used in pediatric practice.
Information on 24-hour ABPM monitors that have successfully passed the independent tests and comply with national standards (Association for the Advancement of Medical Instrumentation and British Hypertension Society [BHS]) can be found on the Internet: however, only two among 25 monitors are recommended for use in pediatric practice.5 The first BHS protocol was published in 1990, and its aim was to standardize the process of verification of monitors for noninvasive BP measurement.6 The protocol was revised in 1993 and received international recognition.7 In 2011, the results of validation of the BPLab® monitor (PetrTelegin, Nizhny Novgorod, Russia) for BP measurement in adult patients were published. According to the obtained results, the BPLab monitor was assigned to the “A/A” accuracy class for systolic and diastolic BP.8 We consider these results as a first phase of the present study.
Materials and methods
Subject selection was based on the requirements of Part II of the 1993 BHS (BHS-93) protocol.7 A group of 30 “older” children (aged 5–15 years according to the BHS-93 protocol) of both sexes (15 boys and 15 girls) of different ages (5 years, n=2; 6 years, n=2; 7 years, n=2; 8 years, n=3; 9 years, n=1; 10 years, n= 3; 11 years, n=2; 12 years, n=5; 13 years, n=2; 14 years, n=3; 15 years, n=5) who had received detailed instructions about the measurement procedure were recruited to participate in this study. Before the start of the study, we had received the approval of the regional scientific ethical committee. All participants and their parents signed their written consent for participation in the study.
Exclusion criteria were congenital heart or blood vessel disease, arrhythmia, pregnancy, and body mass index less than the 10 percentile or more than the 90 percentile according to sex and age. Subjects with Korotkoff sounds tending towards zero during examination were excluded from participation.
Test measurement conditions
According to the BHS-93 protocol, the results of BP measurement obtained by a qualified operator using the BPLab device were compared with BP values measured using the Korotkoff auscultatory method (Phase I for systolic BP and Phase V for diastolic BP) simultaneously by two experts, both of whom were pediatric cardiologists with over 25 years of experience, who had completed a noninvasive BP measurement standardization training course. Thus, there were three observers: the first observer measured BP using the test unit (BPLab device), and the second observer and third observer measured BP using the Korotkoff auscultatory method.
Measurements were taken in the morning in a comfortable setting (including ambient temperature 23°C–25°C and no irritating sounds) after 10 minutes of relaxation in a seated position. Stimulants (tea and coffee) were not allowed 8 hours before measurement; patients were only allowed a light breakfast not later than half an hour prior the test. The children were require not to have taken any medications within the 3 days before the test.
BP measurements were performed on the nondominant arm by two experts simultaneously using a high-quality stethoscope with one head and two headbands and two individual calibrated sphygmomanometers. The cuff pressure deflation rate was 2 mmHg per second. The cuff size depended on the patient’s arm circumference.
Measurement schedule
The BP of each patient was measured nine times, alternating between the experts and the test device according to the following schedule: measurement A, expert; measurement B, instrument; measurement 1, expert; measurement 2, instrument; measurement 3, expert; measurement 4, instrument; measurement 5, expert; measurement 6, instrument; and measurement 7, expert.
BP was measured on the nondominant arm. The interval between measurements was 30–60 seconds. After the experts had obtained their BP values, they made records in their own tables.
Three measurement error values were calculated for each patient (for systolic BP and diastolic BP, separately) according to the following formulae:
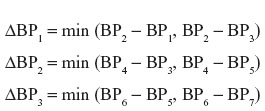
where BPn is a BP measurement result corresponding to the measurement number n. Measurement A was used to assign a patient to each group according to their BP level (Table 2); measurement B was treated as a calibration measurement. Discrepancies in systolic and diastolic BP measurements (n=180; 90 for each expert) were subsequently analyzed according to the criteria specified in the BHS-93 protocol.
Results
The statistical distribution of the patients’ clinical parameters is given in Table 1, and their distribution according to BP level and arm circumference is shown in Table 2.
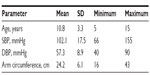  | Table 1 Patients’ clinical profile parameters (15 boys, 15 girls) |
  | Table 2 Grouping of patients according to different parameters |
Detailed results of the data analysis are given in Table 3 in the way recommended by the BHS-93 protocol.7 The number of measurement error values which are within the ranges envisaged by the protocol is indicated in the form of a percentage ratio in relation to the total number of measurements.
Figures 1 and 2 are a graphical illustration of discrepancies in each measurement depending on the relevant BP value (separately for systolic BP and diastolic BP). As recommended in the BHS-93 protocol, the figures show only the results of comparison with the expert who obtained closer results (observer 1 for systolic BP, observer 1 for diastolic BP).
  | Figure 1 Plot of pressure difference between the better observer and the test device, and the mean pressure in 30 patients for SBP (n=90). |
  | Figure 2 Plot of pressure difference between the better observer and the test device, and the mean pressure in 30 patients for DBP (n=90). |
Discussion
ABPM is the method of choice for the diagnosis and control of arterial hypertension in both pediatric and adult patients.1–4 Although ABPM is widely used in children, there are still some open questions connected with normative data sets in children and appropriate validation of devices for use in the pediatric population.
The BPLab ABPM device is known to have accuracy class “A/A” for measuring peripheral arterial BP in adults.8 Moreover, the device was clinically validated for measuring central aortic pressure and parameters of arterial stiffness,9–11 which provides normative data for arterial stiffness and central BP indices.12 However, validation of the accuracy of the device in the pediatric population has not been performed as yet. Thus, the aim of this study was to validate the accuracy of the device for measuring peripheral arterial BP in “older” children (aged 5–15 years).
The results of this study suggest that the BPLab ABPM device has passed the test in a special group of patients, ie, “older” children (5–15 years) in accordance with the requirements of the BHS-93 protocol, and may be assigned to the “A/A” accuracy class as per the above-mentioned protocol. The tested device may be recommended for extensive pediatric use.
Disclosure
The authors report no conflicts of interest in this work.
References
Flynn JT. Impact of ambulatory blood pressure monitoring on the management of hypertension in children. Blood Press Monit. 2000;5:211–216. | |
Koch VH, Colli A, Saito MI, et al. Comparison between casual blood pressure and ambulatory blood pressure monitoring parameters in healthy and hypertensive adolescents. Blood Press Monit. 2000;5:281–289. | |
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. | |
Lurbe E, Cifkova R, Cruickshank K, et al. Management of high blood pressure in children and adolescents; recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719–1742. | |
Dabl Educational Trust. Devices for Ambulatory Blood Pressure Measurement. Available from: http://dableducational.org/sphygmomanometers/devices_3_abpm.html#AbpmTable. Accessed December 29, 2014. | |
O’Brien E, Petrie J, Littler WA, et al. The British Hypertension Society protocol for the evaluation of automated and semi-automated blood pressure measuring devices with special reference to ambulatory systems. J Hypertens. 1990;8:607–619. | |
O’Brien E, Petrie J, Little WA, et al. The British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11 Suppl 2:S43–S62. | |
Koudryavtcev SA, Lazarev VM. Validation of the BPLab® 24-hour blood pressure monitoring system according to the European standard BS EN 1060-4:2004 and British Hypertension Society protocol. Med Devices (Auckl). 2011;4:193–196. | |
Rogoza AN, Kuznetsov AA. Central aortic blood pressure and augmentation index: comparison between Vasotens® and SphygmoCor® technology. Research Reports in Clinical Cardiology. 2012;3:27–33. | |
Posokhov IN. Pulse wave velocity 24-hour monitoring with one-site measurements by oscillometry. Med Devices (Auckl). 2013;6:11–15. | |
Kotovskaya YV, Kobalava ZD, Orlov AV. Validation of the integration of technology that measures additional “vascular” indices into an ambulatory blood pressure monitoring system. Med Devices (Auckl). 2014;7:91–97. | |
Kuznetsova TY, Korneva VA, Bryantseva EN, et al; Vasotens Registry Collaborators. The 24-hour pulse wave velocity, aortic augmentation index, and central blood pressure in normotensive volunteers. Vasc Health Risk Manag. 2014;10:247–251. | |
De Swiet M, Fayers P, Shinebourne EA. Blood pressure in first 10 years of life: the Brompton study. BMJ. 1992;304:P23–P26. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

