Back to Journals » Breast Cancer: Targets and Therapy » Volume 7
Triple negative breast cancer: an Indian perspective
Authors Akhtar M, Dasgupta S, Rangwala M
Received 25 March 2015
Accepted for publication 20 May 2015
Published 14 August 2015 Volume 2015:7 Pages 239—243
DOI https://doi.org/10.2147/BCTT.S85442
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pranela Rameshwar
Video abstract presented by Professor Doctor Murtaza Akhtar
Views: 7558
Murtaza Akhtar, Subhrajit Dasgupta, Murtuza Rangwala
Department of Surgery, NKP Salve Institute of Medical Sciences and Research Centre, Nagpur, Maharashtra, India
Introduction: Breast cancer is the most common female cancer in the world. Triple negative breast cancer (TNBC) is a recently identified biological variant with aggressive tumor behavior and poor prognosis. Data of hormonal status from the Indian population is scarce due to financial constraints in performing immunohistochemistry evaluation. The present study aims to prospectively analyze receptor status of all breast cancer patients and identify TNBC and compare their clinical profile and short term survival with other non-TNBC group.
Materials and methods: All cytologically and histopathologically confirmed cases of carcinoma breast were prospectively enrolled. In a longitudinal study at tertiary care hospital in central India based on the hormonal status, they were further divided into TNBC and other groups. Comparison of risk factors, clinical profile and short-term survival was carried out.
Results: A total 85 patients were enrolled and of them 37 (43.7%) were TNBC. On comparing risk factors ie, age, age at menarche, total reproductive age, age at first child birth, and menopausal status – no statistical significance was observed between the TNBC and non-TNBC groups. But on comparison of clinical profile TNBC tumors were significantly large with majority of patients presenting as locally advanced breast cancer (83%). No statistical difference was observed in axillary lymph node status between two groups. TNBC tumors were histologically more aggressive (grade 3) compared to other groups. No statistically significant difference was observed in short term overall survival but all three deaths were observed in the TNBC group only and two local recurrences after surgery were observed in the TNBC group.
Conclusion: TNBC forms a large proportion of carcinoma breast patients in a central Indian scenario and needs more research to identify appropriate treatment planning considering aggressive histology and advanced presentation.
Keywords: triple negative breast cancer, TNBC, hormone receptor, breast cancer, ER, PR, HER2neu
Introduction
Breast cancer is the most common malignancy worldwide accounting for 21% of all cancers1 and is the most common cancer among females in urban India.2,3 It is a heterogeneous disease of different biological subtypes recognized by gene expression study using DNA microarray.4 These biological subtypes are known to have varied clinicopathological and molecular features having prognostic and therapeutic implications. Hormone receptor analysis is now an established procedure in routine management of breast cancer but the cost of evaluation and non-affordability are key concerns in performing hormone receptor analysis in an Indian scenario. With increasing prevalence of locally advanced breast cancer (LABC) and aggressive tumors it is a good rationale to evaluate hormonal status of breast cancer in central India as there is paucity of hormone receptor data. Triple negative breast cancer (TNBC) is a recent concept and hot topic for research. It is also associated with aggressive tumors, seen in a younger age group, with shorter disease free survival. The present study aims to establish the hormone receptor status of patients presenting in a tertiary care hospital of central India and to determine the prevalence of TNBC and its clinical and biological behavior as well as its comparison with non-TNBC patients.
Materials and methods
All histopathologically and cytologically confirmed cases of breast cancer were prospectively enrolled in a longitudinal study at a tertiary care hospital in central India between 2012 and 2014. The present study had an ethical clearance from the Institutional Ethics Committee of the institute and patients were recruited after obtaining an informed consent in local dialect. Using a case sheet, demographic details, risk factors, clinical profile, and staging of disease was recorded. Confirmation of diagnosis was done by fine needle aspiration cytology and trucut biopsy in LABC cases. Metastatic work-up was done for all patients which included ultrasonography of abdomen and pelvis and chest X-ray and Tc99 bone scan in selected cases. Based on the clinical examinations and measurement of breast lump by Vernier caliper and systemic investigations, patients were categorized into three groups, 1) early breast cancer (EBC), 2) LABC, and 3) advanced breast cancer. The patients with EBC were treated with modified radical mastectomy followed by six cycles of adjuvant chemotherapy with cyclophosphamide, Adriamycin, and 5-Fluorouracil (CAF) followed by hormonal therapy depending on hormone receptor and menopausal status. All LABC patients underwent trucut biopsy for hormone receptor study before starting chemotherapy. Operable LABC patients were subjected to modified radical mastectomy and inoperable LABC patients received neoadjuvant chemotherapy, 2–3 cycles of CAF. Response to chemotherapy was assessed by measuring the lump with Vernier calipers before initiating the next chemotherapy cycle and was assessed using RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1. Those patients who showed clinical response by decrease in size of lump were given three cycles of neoadjuvant chemotherapy (CAF) followed by modified radical mastectomy, followed by three cycles of adjuvant chemotherapy (CAF). Those patients showing no clinical response after two cycles underwent modified radical mastectomy followed by six cycles of adjuvant chemotherapy (taxanes). Patients with positive surgical margins received local radiotherapy. Based on hormone receptor status and menopausal status patients were started on hormonal therapy.
Hormone receptor status analysis was carried out on formalin fixed paraffin block embedded tissue sections. Estrogen receptor and progesterone receptor were considered positive if >1% tumor cell nuclei were immunoreactive and negative if it was otherwise. To establish HER2 status we used US Food and Drug Administration (FDA) approved Hercep test guidelines5 (0 and 1 is negative, 2+ is borderline, 3+ is positive). Based on immunohistochemistry findings the cases were divided into two categories. TNBC and others. Comparison of both group parameters was done using SPSS version 16 (SPSS Inc., Chicago, IL, USA). Continuous variables, after checking normality of data, were compared using Student’s t-test while categorical variables were evaluated using Pearson’s chi-squared or Fisher’s exact test as deemed appropriate. Multivariate analysis was carried out using Cox regression model. Due to short duration of follow-up survival analysis was not carried out.
Follow-up of patients was done every 3 months to look for locoregional recurrence, chest X-ray and ultrasonography of abdomen and pelvis were done to rule out metastasis.
Results
A total of 85 patients were recruited prospectively based on selection criteria from June 2012 to June 2014. The mean age of the patients was 50.01±11.592 years with a range of 25–75 years and of them 50 (58.8%) were postmenopausal while 35 (41.2%) were premenopausal.
Based on hormone receptor status evaluation, 37 patients were classified as TNBC and the remaining 48 patients were allocated to the other group having varied hormone receptor status. The mean age of TNBC group was 48.25 years as compared with the other group which was 51.25 years but it was statistically not significant (P=0.26). Menopausal status evaluation showed more premenopausal patients in the other group compared to TNBC group and the difference was statistically significant P<0.001 (Table 1).
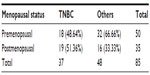  | Table 1 Showing comparison of menopausal status |
On comparing the two groups for risk factors using univariate analysis, age of these patients, age at menarche, total reproductive age, age at first child birth, there were no statistically significant differences between the two groups (Table 2).
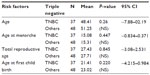  | Table 2 Comparison of risk factors in TNBC and others |
The duration of presentation was 7.5 months in TNBC group compared with 6.8 months in the other group and the difference was statistically insignificant (P>0.01), whereas the size of the tumor was larger in the TNBC group ie, >5 cm in 31 patients out of 37 patients, this was statistically significant on comparison with the other group (Table 3).
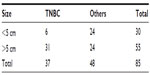  | Table 3 Showing comparison of tumor size |
After excluding the advanced breast cancer group, two groups were compared as LABC and EBC, there was a significantly higher number of patients with LABC in the TNBC group suggestive of locally advanced and aggressive disease (P=0.001) (Table 4).
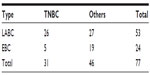  | Table 4 Showing comparison of disease stage |
Positive axillary lymph nodes were observed in 72.97% in TNBC compared to 60.41% in the other group but this difference was not statistically significant (Table 5).
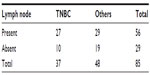  | Table 5 Showing comparison of axillary lymph node status |
On comparing the two groups for Nottingham histological grading of the tumor it was observed that tumors in the TNBC group were higher than grade 3 as compared to others and this difference was statistically significant (Table 6).
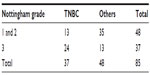  | Table 6 Showing comparison of Nottingham histological grade |
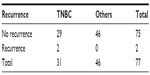
On carrying out multiple logistic regression analysis, Nottingham grade and LABC was statistically associated with TNBC, suggesting aggressive behavior of TNBC. On further analysis, local recurrence was observed in two of the 37 TNBC patients compared to none in the other group. This difference was statistically not significant (Table 7) and overall short-term survival showed three deaths in TNBC compared to none in the other group but this difference was also statistically not significant (Table 8).
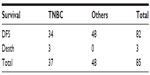  | Table 8 Showing comparison of short-term survival |
Discussion
There is an increasing burden of breast cancer worldwide and in India it is also a cause of concern to health providers and is an important area of research. Introduction of newer technological methods giving insights into tumor biology is also an important area of research solely because of paucity of Indian data and a high prevalence of LABC. The present research was aimed at identifying biologically aggressive tumors with the long-term aim of developing therapeutic strategies and predicting outcomes.6 The present study is a prospective evaluation of the presence of triple negative cancer in a central Indian population and its comparison with risk factors, clinical presentation, and short-term outcome of other hormonal status malignancies.
The literature review shows TNBC accounts for 15% of all breast cancer.7–9 More frequently observed and with worst prognosis in young black women.10,11 In the present study this prevalence was 43.5% of all breast cancers and this is consistent with persistently higher prevalence quoted in Indian populations and ethnic groups (Table 9).12–14
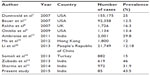  | Table 9 Showing prevalence of TNBC worldwide |
Literature from India and the rest of the world showed TNBC was observed in a younger population15 but the present study showed no significant age difference when compared with other groups and this is consistent with only one study from Turkey.16
On comparing the tumor size between the two groups, it was observed in the present study that tumors in the TNBC group were larger in size (more than 5 cm) as compared to the other group and this finding was quite consistent with the literature.16,17 When this was further compared by staging it was evident that a large number of patients in the TNBC group had LABC, suggesting aggressiveness of the malignancy. Duration of presentation in both groups was similar. Considering that the duration of presentation in both groups were similar, increased occurrence of LABC in TNBC indirectly suggests aggressiveness of the disease. In TNBC there is controversy regarding axillary lymph node metastasis. Lymph node-negative breast cancer is widely reported in the literature but this is contrary to our study results with 73% TNBC patients showing involvement of axillary lymph nodes. These findings confirm results from a study from North East India.12
When evaluating the grade of tumors using the Nottingham grading score, there was a difference noted in tumors of the TNBC group compared to the other group, as the majority of TNBC tumors were of higher grades. These results are consistent with available literature.18–21
Though the present study also looked at the overall survival and local recurrence between the two groups with median survival period of 24 months, there was no significant difference noted between the two groups. However 8.1% patients in the TNBC group succumbed to breast cancer and there was 6.4% local recurrence which is quite significant clinically. This 2-year period is too short to comment on disease free and overall survival.
To conclude, this was a prospective study evaluating the hormone receptor status of breast cancer patients in central India. However, we tried to identify a subgroup of malignancy ie, triple negative phenotype which is a marker of basal type cell carcinoma. The prospective study also helped us to correlate the clinical status and risk factors which had no effect on tumor biology. Triple negative cancer is commonly a prevalent phenotype in patients of central India accounting for 43% of all breast cancer with high grade large tumors and early local recurrence and poor prognosis which needs a definitive review of treatment strategy as TNBCs do not respond to hormone therapy.
The only limitation of the study is its small sample size but it still gives insight into biological typing of breast cancer in central India which comprises of a non-White ethnic groups and clinical correlation with LABC with higher grades of histopathology.
Disclosure
The authors have no conflicts of interest in this work.
References
World Health Organization [homepage on the Internet]. The global burden of disease: 2004 update. WHO. Available from: http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/. Accessed June 8, 2015. | |
Consolidated Report of Hospital Based Cancer Registries 2001–2003. National Centre for Disease Informatics and Research National Cancer Registry Programme (Indian Council of Medical Research); 2007. Available from: http://www.ncrpindia.org/Reports/HBCR_2001_03.aspx. Accessed June 8, 2015. | |
Consolidated Report of Population Based Cancer Registries 2001–2004. National Centre for Disease Informatics and Research National Cancer Registry Programme (Indian Council of Medical Research); 2006. Available from: http://www.ncrpindia.org/Reports/PBCR_2001_2004.aspx. Accessed June 8, 2015. | |
Perou CM, Sorlie T, Eison MB, et al. Molecular Portraits of human breast tumours. Nature. 2000;406(6797):747–752. | |
Wolff A, Hammond ME, Schwartz J, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. | |
Cummings MC, Chamber R, Simpson PT, Lakhani SR. Molecular classification of breast cancer. Is it time to pack up our microscopes? Pathology. 2011;43(1):1–8. | |
Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):R6. | |
Rakha EA, Tan DS, Foulkes WD, et al. Are triple negative tumours and basal-like breast cancer synonymous? Breast Cancer Res. 2007;9(6):404. | |
Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1–2):4–13. | |
Chutstecka Z. Survival Disadvantage Seen for TNBC. Medscape Medical News; 2008. Available from: www.medscape.com/viewarticle/554234. Accessed April 10, 2015. | |
Reynolds S. Spotlight: triple –negative breast carcinoma disproportionately affects African American and Hispanic women. National Cancer Institute Cancer Bulletin. 2007;4:22. Available from: http://www.cancer.gov/ncicancerbulletin/archive/2007/072407. Accessed April 10, 2015. | |
Sharma M, Sharma JD, Sarma A, et al. Triple Negative Breast Cancer in People of North East India: Critical Insights Gained at a Regional Cancer Centre. Asian Pac J Cancer Prev. 2015;15(11):4507–4511. | |
Ambroise M, Ghosh M, Mallikarjuna VS, Kurian M. Immunohistochemical profile of breast cancer patients at a tertiary care hospital in South India. Asian Pac J Cancer Prev. 2011;12(3):625–629. | |
Zubeda S, Kaipa PR, Shaik NA, et al. HER-2/neu status: a neglected marker of breast cancer patients in India. Asian Pac J Cancer Prev. 2013;14(4):2231–2235. | |
Iwase H, Kurebayashi J, Tsuda H, et al. Clinicopathological analyses of triple negative breast cancer using surveillance data from the Registration Committee of the Japanese Breast Cancer Society. Breast Cancer. 2010;17(2):118–124. | |
Somali I, Ustaoglu BY, Tarhan MO, et al. Clinicopathologic and demographic evaluation of triple- negative breast cancer patients among a turkish patient population: a single center experience. Asian Pac J Cancer Prev. 2013;14(10):6013–6017. | |
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–1278. | |
Carey LA, Perou CM, Livsay CA, et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA. 2006;295(21):2492–2502. | |
Ma KK, Chau WW, Wong CH, et al. Triple negative status is a poor prognostic indicator in Chinese women with breast cancer: a ten year review. Asian Pac J Cancer Prev. 2012;13(5):2109–2114. | |
Li CY, Zhang S, Zhang XB, et al. Clinicopathological and prognostic characteristics of triple-negative breast cancer (TNBC) in Chinese patients: a retrospective study. Asian Pac J Cancer Prev. 2013;14(6):3779–3784. | |
Patil VW, Singhai R, Patil AV, Gurav PD. Triple-negative (ER, PgR, HER-2/neu) breast cancer in Indian women. Breast Cancer (Dove Med Press). 2011;3:9–19. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.