Back to Journals » Journal of Pain Research » Volume 7
Treatment of uncomplicated hemorrhoids with a Hemor-Rite® cryotherapy device: a randomized, prospective, comparative study
Authors Guindic LC, Frank P
Received 17 January 2013
Accepted for publication 3 July 2013
Published 16 January 2014 Volume 2014:7 Pages 57—63
DOI https://doi.org/10.2147/JPR.S42872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Luis Charúa Guindic
Coloproctology Unit, General Hospital of México, México City, México
Abstract: Hemorrhoids are one of the most common ailments known. Often described as "varicose veins of the anus and rectum", hemorrhoids are enlarged, bulging blood vessels in, and about the anus and lower rectum. About 75% of people will have hemorrhoids at some point in their lives. This paper shares the results from the clinical evaluation conducted to study effects of cryotherapy in treating uncomplicated hemorrhoids. The device used in the study is based on topically-applied cold therapy which can produce vasoconstriction in the tissues, tissue hypoxia, analgesia, and muscle relaxation. Cryotherapy was shown to be statistically similar or superior to proctology ointment in some of the parameters studied such as reduction of pain and hemorrhage. Overall it was observed that cryotherapy device contributes to improving the quality of life of patients with hemorrhoids.
Keywords: cryotherapy, hemorrhage, hemorrhoids, analgesic
Introduction
Hemorrhoidal disease has been one of the most common human ailments. Hippocrates (460–377 BC) in the Hippocratic Corpus made reference to hemorrhoids and proposed medical and surgical treatments. Galen (131–201 AD) in De Medicina described hemorrhoid ligation and extirpation of hemorrhoids, as well as their complications. Maimonides (1235–1305 AD) in his Treatise on hemorrhoids noted the importance of diet and detailed a number of treatments to alleviate hemorrhoidal symptoms, including “fatty chicken broth.” He also recommended medicines to be applied as suppositories, creams, or enemas to eliminate or prevent symptoms.1
The term “hemorrhoids” comes from the Greek word “haimorrhoides”, which means: haima(blood or bleeding) and rhoos(flow).2 It is difficult to have an exact measure of the prevalence of this disease, but we can state with confidence that in Mexico at least 5% of the general population have hemorrhoid-related symptoms. Rare before the age of 20 years, hemorrhoidal symptom frequency increases with age; probably 50% of adults 50 years or older have experienced hemorrhoidal symptoms at some time in their lives.3,4
The etiology of hemorrhoids has never been proven. For many years, it was thought that hemorrhoids were made up of dilations in blood vessels of the upper and lower hemorrhoidal plexus, but the real explanation may not be so simple. In general, it has not been possible to identify a particular cause that triggers the condition, and it is commonly accepted that certain factors can play a role in the process in a given patient. The most consistent of these is constipation: with greater effort made during evacuation, congestion occurs in the anal cushions. With constant straining, the supports of these cushions become distended and cause hemorrhoidal prolapse below the anorectal junction and outside the anal canal. However, it is important to note that not all patients with hemorrhoidal disease are constipated. Therefore, alcohol, spicy foods, constipation, diarrhea, pregnancy, occupation, sedentary life style, and possibly other factors should be considered as predisposing, not as etiologic, factors.
To promote better understanding of hemorrhoidal disease, the Coloproctology Unit of the Gastroenterology Department, Hospital General de México, classifies hemorrhoids as internal or external. Patients usually have a combination of both types; only a minority of patients has only one type. Internal hemorrhoids are considered arbitrarily as small, medium, or large, and are covered by skin. They are located at the lower end of the rectum, are covered by mucosa, and are clinically graded. Grade I hemorrhoids are those that bleed. Grade II hemorrhoids are those that bleed and extend throughout the anal canal, but do not project outside the canal, and spontaneously retract. Grade III are hemorrhoids that bleed and prolapse outside the anus during defecation and retract spontaneously or require manual retraction. Finally, grade IV hemorrhoids are those that bleed and are permanently prolapsed.3
Current treatment for hemorrhoidal disease should be tailored individually for each case; possible treatments are medical, nonsurgical alternative, and surgical treatment.4 Medical treatment should be used on all patients with hemorrhoidal symptoms, but especially in patients with Grade I or II hemorrhoidal disease, treatment is based on regularizing bowel habits. To do this, the patient should be instructed on the type of diet to follow, primarily a diet rich in fiber, free of irritants, and with an adequate amount of fluids. In a small number of cases, hydrophilic agents such as seeds of psyllium plantago, among other seeds, may be added.1–4 Whereas ointments or suppositories to treat hemorrhoidal disease are somewhat useful, most provide only symptomatic treatment but not a long-term solution. The classic treatment for this disease has been surgical, but postoperative pain, hospital cost, time away from work, and patient reluctance have led to the creation of procedures that avoid these drawbacks. Nonsurgical alternatives for hemorrhoidal disease include elastic band hemorrhoid ligation, sclerotherapy, cryotherapy, bipolar coagulation (BICAP; Circon-Acmi, Stamford, CT, USA), galvanic current (Ultroid®, Vascular Technologies, Inc, Tampa, FL, USA), and photocoagulation with infrared radiation.3–13
The Hemor-Rite® cryotherapy device (Med-Rite Laboratories, Dallas, Texas, USA), anatomically designed for the application of cold therapy (cryotherapy) directly on external and internal hemorrhoidal masses, is a new alternative for treatment of uncomplicated hemorrhoidal disease. The purpose of the present study is to evaluate the Hemor-Rite® device and to compare it to proctology ointment (PO) (100 g contains 5 g of tribenoside and 2.12 g of lidocaine hydrochloride) and placebo (petrolatum).
Materials and methods
Study participants and groups
The participants in this randomized, prospective, longitudinal, balanced multiple-dose comparative and descriptive study of three possible treatments were patients who were initially seen by the lead investigator of this study at the Coloproctology Unit of the Gastroenterology Department, Hospital General de Mexico from October 2011 to May 2012, and who met the criteria for inclusion. The clinical files of 108 patients were reviewed, but only 90 met the criteria for inclusion. Of these 90 patients, 53 (58.89%) were male and 37 (41.11%) were female, with an overall average age of 44 years (average 42.58 for males and 46 for females) with an age range of 21 to 69 for men and 26 to 85 for women.
Inclusion criteria were as follows: individuals between the ages of 18 and 65 years, weighing more than 60 kg, patients who were seen initially at the Coloproctology Unit of the Gastroenterology Department, Hospital General de México or the private office of the lead investigator for symptoms of uncomplicated hemorrhoidal disease without an additional anorectal pathology such as anal fissure or fistula, abscess, or perianal lesions; and patients who had undergone proctological examination by the lead investigator of the clinical trial or by a resident from the Coloproctology Specialization Program at the hospital, to determine whether the patient presented a clinical picture indicating hemorrhoidal disease. Exclusion criteria were as follows: patients with another anorectal condition such as anal fissure or anal fistula, among others; patients who had no symptoms of external or internal hemorrhoids at the initial consultation; and patients who had previously undergone anorectal surgery.
The patients were randomly assigned to one of the three groups: members of the first group received the Hemor-Rite® cryotherapy device, which was applied as directed three times per day for 8 minutes for 6 days. Patients in the second group were treated with PO applied three times per day for 6 days, and the third group was treated with the placebo applied three times per day for 6 days. Three of the 37 women included in this study were pregnant; one was 26 weeks pregnant, another 29 weeks, and another 34 weeks. Two of the pregnant women were included in the Hemor-Rite® cryotherapy device group, and the other in the placebo group. Informed consent was obtained from all patients. We took a complete clinical history from all patients and performed a proctological examination that included inspection of the anoperineal region, manual examination of the rectum, and examination with a 25 cm rigid proctosigmoidoscope.
Elimination criteria were: patients who did not correctly apply the Hemor-Rite® cryotherapy device the correct number of times, or for the right length of time; those who did not apply the PO or the placebo three times per day for six days; and finally, patients who did not come to their checkup 7 days after the start of treatment. Thirty-two patients were eliminated.
The following patient-characteristic variables were analyzed: age, sex, reason for consultation, duration of disease, grade, treatment used, bleeding, anal pain and itching. Pain was rated based on a visual analog scale for pain, from 0 for absence of pain to 10 for extremely severe pain; it was assessed three times per day for 6 days. Rectal bleeding was classified as absent, mild, moderate, and severe, and was assessed three times per day for 6 days. Classifications of pain and bleeding were recorded on an information form filled out by the patient.
Structure and application of the medical device
The Hemor-Rite® cryotherapy device is anatomically designed for the direct application of cold therapy to the external and internal hemorrhoidal masses. It was designed with consideration of human anatomy and medical concepts for treatment of this medical condition. It has been shown that direct application of cold can provide immediate relief of pain, itching, and inflammation due to the vasoconstrictive and analgesic properties of this medical device.
The device is made up of two plastic pieces. The crown, which is inserted into the rectum, has a curved tip to facilitate insertion. The base is longer than it is wide. The two pieces (crown and base) meet at the base and are joined there. The crown is 4.56 cm high. The maximum diameter is 3.25 cm. The base is 0.71 cm high and 6.19 cm long (Figure 1). The device as supplied is packaged in a plastic case and includes two bottles of lubricant to facilitate insertion. Before delivery to the patient in our study, the device was stored at room temperature and required no further preparation. The patient was instructed to place the device in the freezer for at least 3 hours immediately after taking it home; at this time, the device is ready to be used.
  | Figure 1 The Hemor-Rite® cryotherapy device (Med-Rite Laboratories, Dallas, TX, USA). |
Results
Pain assessment
An analysis of repeated samples was performed to explore the effect of the three treatments on the dependent variable of pain assessment. We also analyzed the effect of sex and the presence, or absence, of pain (before starting treatment) on the dependent variable. We used age, type of internal hemorrhoid (grade I, II, III, or IV), type of external hemorrhoid (small, medium, or large), and baseline pain assessment as covariates. Treatment group, sex, type of internal hemorrhoid, age, and baseline pain assessment proved to be statistically significant (P < 0.05). The marginal mean estimated per group and sex is shown in Tables 1 and 2 and in Figures 2 and 3.
  | Table 1 Size of the effect per treatment group and sex |
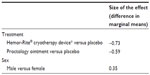  | Table 2 Size of the effect corresponding to treatment group and sex |
  | Figure 2 Day-by-day pain comparison of the various treatments used. |
  | Figure 3 Day-by-day pain comparison of the different treatments used in the three applications per day. |
Bleeding assessment
The bleeding variable was analyzed using generalized estimating equations to evaluate the association between selected factors and the dependent variable of the presence of moderate to severe bleeding, whereas the covariates were checked and adjusted for repeated samples. The factors explored were treatment group, sex, presence or absence of pain (prior to start of treatment), type of internal hemorrhoid (Grade I, II, III, or IV), type of external hemorrhoids (small, medium, or large), number of days, and applications (during the day); the covariates were age, baseline bleeding, and baseline pain assessment. Baseline bleeding, number of days of application and treatment group were statistically significant (P < 0.05). The odds ratios of the significant variables are shown in Tables 3 and 4.
  | Table 3 The estimated probabilities of moderate to severe bleeding for the three treatment groups |
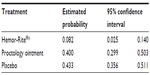  | Table 4 The probabilities of moderate to severe bleeding for the three treatment groups with a confidence interval of 95% |
We also compared the possibility of moderate or severe bleeding occurring in the three treatments studied. We found moderate or severe bleeding to be significantly lower in patients who used Hemor-Rite® cryotherapy device (Figure 4).
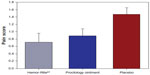  | Figure 4 Final pain score for the three treatment alternatives. |
Additional assessments of the cryotherapy device
The 30 patients (100%) in the Hemor-Rite® cryotherapy device group studied experienced no discomfort, injuries, or symptoms from the insertion or temperature of the device.
Discussion
Data published by the National Center for Disease Staging in the United States indicate that 10,000,000 people suffer from hemorrhoids in the United States alone.14 It is impossible to know its incidence in Mexico, but the Coloproctology Unit of the Gastroenterology Department, Hospital General de México accounts for 30% of the patients who are seen for the first time, with a similar prevalence in both sexes, which is consistent with reports from other studies.1,15,16
The course of hemorrhoidal disease in patients at the time of the initial consultation was usually more than 24 months; this can be attributed to a number of factors, such as economics, embarrassment, lack of information, self-medication, or fear, which prevent patients from seeing a physician in a timely manner. Consistent with data reported in the literature, the grades of hemorrhoidal disease that appeared most frequently were I and II.1,3,15,16
As was the opinion of the lead investigator of this study, most authors consider that the symptoms of this disease can be controlled with appropriate medical management.1,3,16 Therefore, the patient should be instructed concerning the type of diet to follow that is essentially a fiber-rich diet free of irritants and with an adequate amount of liquids. In a very limited number of cases, hydrophilic agents such as seeds of psyllium plantago or gentle laxatives such as lactulose or polyethylene glycol are added. The usefulness of ointments, creams, or suppositories for proctological use is relative. In general, the active ingredients of these medicines temporarily improve symptoms as results of their anesthetic, analgesic, and anti-inflammatory effects.
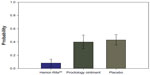  | Figure 5 Probability of moderate or severe bleeding by treatment group (95% confidence interval). |
The Hemor-Rite® cryotherapy device was created to improve upon these principles. This device, based on cold therapy for topical application, can produce vasoconstriction, tissue hypoxia, analgesia, and muscle relaxation. Cold-related vasoconstriction causes heat loss from the skin and deep tissues. This vasoconstriction occurs by direct action and spinal reflex action; it decreases blood supply and is considered the main mechanism for reducing swelling and bleeding after trauma. Vasoconstriction also decreases edema under inflammatory response, thereby decreasing the initial severity of hemorrhoids.
Cold inhibits the release of chemical mediators such as histamine and reduces oxygen needs because of lower metabolic demand. These two factors, together with vasoconstriction, explain the reduction of inflammation. Application of cold can either cause pain or relieve pain. Cryotherapy thus plays a dual role in regard to its relationship to pain, and the mechanisms involved in this relationship have not been explained. Application of cold relieves pain by interrupting the pain-spasm-pain cycle that was triggered by the hemorrhoids and thereby acts to reduce pain and muscle spasm. Theories that attempt to explain this action are based on the decrease in sensory impulses, reflex mechanisms, and decrease in muscle spasm. Application of cold produces a muscle-relaxing effect. The most significant reduction of spasticity occurs while the cold is being applied but can persist for several hours. This explains why patients with hemorrhoidal symptoms had longer periods of relief after application of the Hemor-Rite® cryotherapy device.
For effectiveness against some of the parameters studied, such as pain and bleeding, the Hemor-Rite® cryotherapy device was found to be statistically similar to or superior to the PO used. The results achieved with Hemor-Rite® cryotherapy device were also statistically superior to the placebo. Another important aspect of this study was the possibility of preventing moderate or severe bleeding, which was found to be significantly less in patients who used the Hemor-Rite® cryotherapy device.
Finally, the patients in the Hemor-Rite® cryotherapy device group showed no indications of injury of any kind from the design of the device or the temperature at which it is used. The device showed no side effects on two pregnant patients who participated in this study and were in the group that was treated with the Hemor-Rite® cryotherapy device.
Conclusion
Based on our analysis, we conclude that the Hemor-Rite® cryotherapy device is a good treatment alternative for patients with symptoms caused by uncomplicated hemorrhoidal disease: the device decreases pain and itching and the likelihood of moderate or severe bleeding. It was also observed that the device did not cause pregnant patients any apparent side effects. However, additional studies of hemorrhoidal treatments are needed to produce results with a higher level of evidence, thus helping to improve the quality of life of patients with hemorrhoidal disease.
Disclosure
Part of the funding to conduct the study was provided by Medrite Laboratories, also a manufacturer and patent holder of the Hemor-Rite® cryotherapy device. The author reports no other conflicts of interest in this work.
References
Maimonides M. Treatise on hemorrhoids. Philadelphia: Lippincott; 1969. | |
Goligher J. irugía de Ano, Recto y Colon. Editorial Masson. 1998; 92–142. | |
Charúa GL. Enfermedad hemorroidaria. In Murguía DD. Gastroenterología y Hepatología Práctica. [Practical Gastroenterology and Hepatology] México, DF, 1a edición. Intersistemas Editores. 1999;15:153–157. Spanish. | |
Avendaño EO. Proctología. Editorial Impresiones Modernas. [Proctology. Modern Editorial Impressions]. S.A. México, D. F. 1968;44–80. Spanish. | |
Burkitt DP. Hemorrhoids, varicose veins and deep vein thrombosis: epidemiologic features and suggested causative factors. Can J Surg. 1975;18:483–488. | |
Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology. 1990;98:330–386. | |
Stelzner F, Staubesand J, Machleidt H. Das Cavernosum Recti-Die Grundlage der Inneren Hammarrhoiden. [The cavernosum recti-The basis of internal hemorrhoids]. Langenbecks Archiv für klinische Chirurgie. 1962;299:302–312. German. | |
Thomson WH. The nature of hemorrhoids. Br J Surg. 1975;62:542–552. | |
Haas PA, Fox TA Jr, Hass GP. The pathogenesis of hemorrhoids. Dis Colon Rectum. 1984;27:442–450. | |
Corman ML. Colon and Rectal Surgery. Fourth Edition. New York:Lippincott-Raven; 1998:147–205. | |
Goldberg SM, Gordon HP, Nivatvongs S. Fundamentos de Cirugía Anorrectal. Hemorroides.[Fundamentals of Anorectal Surgery for Hemorrhoids]. México, D F: Editorial Limusa; 1990;103–121. | |
Jorgen J, Bach S, Stübinger SH, Bock JU. Excision of thrombosed external hemorrhoid under local anesthesia. Dis Colon Rectum. 2003;9:1226–1231. | |
Barron J. Office ligation treatment of hemorrhoids. Dis Colon Rectum. 1963;6:109–113. | |
Charúa GL, Chirino PAE, Navarrete CT, et al. Manejo Alternativo no Quirúrgico de la Enfermedad Hemorroidaria.[Non-surgical alternative in management of hemorrhoidal disease]. Rev Gastroenterol Méx. 2005;7:136–142. Spanish. | |
Neiger A. Hemorrhoids in everyday practice. Proctology. 1979;2:22–28. | |
Charúa GL, Avendaño EO, Hernández CF. La fotocoagulación por rayos infrarrojos en el tratamiento de la enfermedad hemorroidaria. [Infrared photocoagulation in the treatment of hemorrhoids]. Rev Gastroenterol Méx. 1998;63:131–134. Spanish. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
