Back to Journals » Drug Design, Development and Therapy » Volume 8
Treatment of port wine stains with pulsed dye laser: a retrospective study of 848 cases in Shandong Province, People’s Republic of China
Authors Shi W, Wang J, Lin Y, Geng J, Wang H, Gong Y, Liu H , Zhang F
Received 24 July 2014
Accepted for publication 18 August 2014
Published 12 December 2014 Volume 2014:8 Pages 2531—2538
DOI https://doi.org/10.2147/DDDT.S71710
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Shu-Feng Zhou
Wenhao Shi,1–3 Jinliang Wang,4,5 Yan Lin,4,5 Jianhui Geng,4,5 Haixia Wang,4,5 Yueqin Gong,4,5 Huaxu Liu,1,4,5 Furen Zhang1–4
1Shandong Provincial Institute of Dermatology and Venereology, Shandong Academy of Medical Sciences, 2Shandong Provincial Key Lab for Dermatovenereology, 3School of Medicine and Life Sciences, University of Jinan-Shandong Academy of Medical Sciences, 4Shandong Provincial Hospital for Skin Diseases, Shandong University, 5Shandong Provincial Medical Center for Dermatovenereology, Jinan, Shandong, People’s Republic of China
Background: Currently, 595 nm pulsed dye laser (PDL) therapy is offered as one of the effective treatments of port wine stains (PWSs). However, the efficacy of PDL differs in different populations.
Objective: The purpose of the study was to investigate the efficacy, and related factors, of 595 nm PDL in the treatment of PWSs in Chinese patients with skin type III to IV.
Methods: A total of 848 cases that were treated with PDL were enrolled and analyzed in this study. An independent dermatologist evaluated these lesions according to the before and after photographs.
Results: The response rate (RR) of all the 848 PWS patients was 69.9%, within which the cure rate was 6.3%. The patients aged ≤1 year had the highest RR (93.9%), whereas those treated after age 50 reacted the worst (RR =25%). We analyzed the anatomical distribution of the lesion and found that the temporal region had the highest lesion clearance (RR =75.3%), while the extremities had the lowest clearance (RR =44.5%). Compared with the patients whose lesion size was larger than 80 cm2, the patients with small lesion size, of 0–20 cm2, had better clinical effect (RR =73.8% vs 53.2%). The reactions of the patients with hyperplastic lesion were worse than those with red patches (RR =36.4% vs 71.7%). As well, increasing treatment numbers could achieve higher clearance rates (P=0.005).
Conclusion: The PDL had a relatively high RR but a low clearance rate in Chinese patients with PWS, although the earlier the intervention, the better was the efficacy. The response of PDL was, not only related to the anatomical area, but also, to the lesion size, type of lesion (ie, the presence of existing hyperplastic lesions), and the number of treatment, all of which are essential for the evaluation of therapeutic effect and acquisition of patients consent before treatment.
Keywords: capillary malformation, laser treatment, curative effect, Chinese patient
Introduction
Port wine stains (PWSs) are benign congenital capillary malformation, which occur in 0.3% of all newborns and mostly appear at the face and neck areas.1,2 PWS lesions are rarely eliminated without intervention, and the vast majority of lesions would worsen with the patient’s age.3,4 Pulsed dye laser (PDL) remains the gold standard of treatment, even now.5 The mechanism of PDL in treating PWS is to destroy capillary malformation by selective photothermolysis.6
There were some related retrospective studies of PDL in the treatment of PWS. In 2002, Ho et al performed research in which 107 patients with PWS were treated with laser (wavelength: 532 nm and 585 nm) and found that Chinese patients had lower sensitivity to laser treatment, with higher complication rates and more treatment sessions required to achieve maximum blanching.7 In 2003, Laube et al performed another study of 12 patients with PWS who were treated with V Beam® PDL (Candela Corp, Wayland, MA, USA). They found the 595 nm V Beam PDL appeared to achieve further lightening of therapy-resistant PWS in the majority of patients (67%), but their results required confirmation in larger studies.8
Therefore, our retrospective study was committed to investigate the efficacy of laser treatment and related factors, in a large sample.
Methods
Patients
In this retrospective study, we collected the information of 1,027 PWS patients who were treated in Laser Department of Shandong Provincial Hospital for Skin Diseases from April 1999 to April 2013. Subject inclusion criteria included: patients with PWS who received 595 nm PDL treatment with no previous treatment history of PDL, where the Fitzpatrick skin classification was III to IV; patients who were treated at least three times with 595 nm PDL (including even a few who were treated about 20 times); treatment interval of 4–6 weeks between every two treatments; photographs of the patient were available to compare the effects before and after treatments; and there was a follow-up period of at least 4 months following the latest 595 nm PDL treatment.
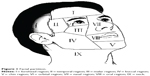
Finally, 848 patients according with the aforementioned conditions were eligible for this study, and informed consent was obtained from all patients. The detailed information of patients was analyzed, including age, anatomical area, the lesion size, presence of existing hyperplastic lesions, and the number of treatment. Among the group, there were 380 patients with PWS having skin lesions in two or more facial regions (Figure 1), and 44 patients had hyperplastic lesion.
Therapy methods
All the cases were treated with flashlamp-pumped PDL (V Beam), with wavelength 595 nm, adjustable pulse width (0.45~40 ms), maximum output energy 6 J, equipped with dynamic cooling device (DCD). The possible parameters for laser treatment: dynamic cooling spray time 30 ms, delay time 20 ms, energy density and pulse width, as determined by the age, skin lesion area, skin type, and immediate reaction after a test pulse. The most commonly selected energy density is 8–12 J/cm2, and the pulse width is 1.5–10 ms. According to the lesion area and tolerance of patients, ELA-Max cream (containing 4% lidocaine) was applied for surface anesthesia before treatment. Then the laser treatment was carried out. Immediate cooling was applied to the treatment area, and some specific skin care products were recommended to the patients after treatment. All the patients were asked to avoid ultraviolet (UV) exposure.
The typical tissue reaction is indicated by mild to moderate purpura, whereas overtreatment and risk of pigmentation is indicated by a charcoal gray color. The goal in treatment was to show an effect without overlap of pulses.
Assessment methods
The clinical assessment of the treatment response was performed by an independent dermatologist in charge who was not involved in the previous treatment. He observed the changes of color, area size, and proliferative lesions of the patient’s “red mole” (PWS) by comparing the pictures of patients before and after treatment, and then gave evaluations using the Physician Global Assessment.9 The Physician Global Assessment includes “poor improvement” (skin recovery: 0%–25%), “moderate improvement” (skin recovery: 26%–50%), “significant improvement” (skin recovery: 51%–75%), and “cure” (skin recovery: 76%–100%). Cure, significant improvement, and moderate improvement were defined as the response rate (RR). The adverse reactions of each patient were recorded, including ache, rash, pigmentation, depigmentation, and formation of scar. The Fitzpatrick skin type classification system was also used and is an assessment of sun sensitivity,10 as follows: I = always burn, never tan; II = usually burn, tan less than average; III = sometimes mild burn, tan about average; IV = rarely burn, tan more than average; V = never burn, tan more than average; VI = never burn, always tan.
All assessment outcomes of the eligible patients with PWS who were treated by 595 nm PDL were recorded.
Statistical analysis
Statistical analysis was performed using SPSS 17.0. For the relationship between related factors (age, anatomical area, lesion size, presence of existing hyperplastic lesions, and number of treatments) and response to laser therapy, we used the Kruskal–Wallis test and bivariate correlation analysis. We also performed the post hoc Scheffe test to compare the data between each group. Rates were compared using the χ2 test. Two-sided P-values <0.05 were considered statistically significant.
Results
Among all 1,027 PWS patients who were treated by PDL in our hospital, 104 patients were not involved in this study as they had fewer than three treatments, 31 patients had previous treatment history of PDL, 28 patients were lost to follow up because of failure in communication, eleven patients were excluded because their photographs were blurry, which made it difficult to distinguish the change of skin lesions, and five patients indicated unequivocally that their information must not be used for any medical study. In summary, a total of 848 patients were enrolled in the analysis.
Among the 848 participating patients, the ages ranged from 38 days to 58 years (mean age =18.7±9.6 years), and males accounted for 45.5% (386/848). Their Fitzpatrick skin types were III to IV. None of the participating patients had received any form of treatment before PDL treatment.
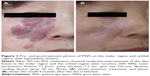
The PWSs treated with the PDL were mainly located on the face (721 patients), followed by neck in 52 patients, trunk in 30 patients, and extremities in 45 patients. Of the patients with PDL on the face, 380 had two or more areas of skin lesions. In the majority of participating patients, the sizes of the skin lesions were within 0–20 cm2 (442 patients). There were 44 patients with hyperplastic lesions, including nodular and thickened lesions (Figure 2).
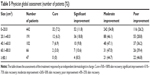
The number of treatments among the 848 patients was 3–20. The mean number of PDL treatments for PWS was 6.2±3.8. The shortest follow-up period from the last PDL treatment was 4 months, and the longest follow-up period was 1½ years (Table 1).
 
|
Table 1 Demographic data |
The Physician Global Assessment showed a cure (76%–100% of PWSs) in 53 (6.3%) of the 848 patients, significant level of improvement or recovery (51%–75%) was scored in 109 (12.9%) of 848 patients, moderate level of improvement or recovery (26%–50%) was found in 430 (50.7%) of 848 patients, and poor level of improvement or recovery (0%–25%) was in 256 (30.1%) of 848 patients (Table 2).
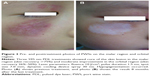
During the follow-up period of the 848 patients, there were 42 patients who had postinflammatory hyperpigmentation that lasted for more than 2 weeks, but all of them had recovered within 3 months, while hypopigmentation occurred in 17 cases (Figure 3).
Association of the PDL effect with the age of PWS patients
The 848 patients were divided into eight groups based on their ages, and the effect of PDL treatment was different between each of the groups. The effect in the youngest group that was ≤1 year old (99 cases) was better than in the other groups (P<0.001); the RR to PDL treatment in this group was 93.9%, and the cure rate was 18.2%. The effect in the oldest group, of 50–60 years (4 cases), was worse than in the other groups (P=0.05); the RR of this group was 25%, and the cure rate was 0% (Table 3).
There was a correlation between the eight age groups and the improvement of PWS after PDL therapy (χ2=70.837, P<0.001 [Kruskal–Wallis test]). Bivariate correlation analysis results (rs=0.281, P=0.000 [two-sided, Pearson]; rs=0.274, P=0.000 [two-sided, Spearman]) showed that these two tests were consistent. The data revealed that there was a relationship between the age of patients and the curative effect to PDL, and the earlier the interventions, the better was the efficacy; however, no significant difference was found between the groups ≤1 year, 6–10 years, 21–30 years, 51–60 years and between the groups 31–40 years, 41–50 years, 51–60 years (P>0.05 [Scheffe post hoc test]).
Association of the PDL effect with the areas of lesions in PWS patients
In the 848 patients with PWS, 380 patients had two or more areas of skin lesions on the face, and the assessments of effect, which was different in the various areas of the face, were recorded for these patients. We divided skin lesions areas into eleven regions, including eight regions on the face, neck, trunk, and extremities and found the best effect existed in the temporal region (90/105) (P=0.01), with RR of 75.3% and cure rate of 8.6%. The worst effect occurred in the extremities (20/45) (P<0.001), with a RR of 44.5% and cure rate of 6.7% (Table 4).
By analyzing the clinical data of patients, we found that there was a relationship between the eleven regions of skin lesions and the improvement of PWS after PDL therapy (χ2=25.488, P=0.004 [Kruskal–Wallis test]). At the same time, bivariate correlation analysis (rs=0.083, P=0.007 [two-sided, Pearson]; rs=0.085, P=0.006 [two-sided, Spearman]) showed that these two tests were consistent. The results suggested that there was a significant difference in the response to PDL among the PWS patients with different areas of lesions; however, there was no significant difference in response to PDL between temporal region, forehead region, chin region, nasal region (P>0.05 [Scheffe post hoc test]).
Association of the PDL effect with the size of skin lesions of PWS patients
The size of the largest skin lesions among the 848 PWS patients was 143 cm2, and the size of the smallest skin lesion was 0.3 cm2. We divided the skin lesion sizes of these patients into five groups. Among the groups, the best effect of PDL treatment was the group of 0–20 cm2 (326/442) (P=0.009), with a RR of 73.8% and cure rate of 7.2%. The worst effect was the group of more than 80 cm2 (25/47) (P=0.011), with a RR of 53.2%, and cure rate of 0% (Table 5).
There was a correlation between the five groups of sizes of skin lesions and the response to PWS (χ2=15.680, P=0.003 [Kruskal–Wallis test]). A bivariate correlation analysis was executed (rs=0.119, P=0.000 [two-sided, Pearson]; rs=0.101, P=0.003 [two-sided, Spearman]), and we found that these two tests were corresponding. Better response to PDL was found in patients with smaller sizes of skin lesions; however, there was no statistically difference between the groups of 40.1–60 cm2, 60.1–80 cm2, and more than 80.1 cm2 (P>0.05 [Scheffe post hoc test]).
Association of the PDL effect with presence of existing hyperplastic lesions in PWS patients
There were 44 patients having hyperplastic lesions among the 848 patients with PWS. Hyperplastic lesions included nodular and thickened lesions. The RR efficiency of nonhyperplastic lesions was 71.7% (576/804), and the cure rate was 6.6% (53/804). However, the RR efficiency of hyperplastic lesions was 36.4% (16/44), and the cure rate was 0% (Table 6).
We found that there was significant difference of the response to PWS between the groups that had or did not have existing hyperplastic lesions (χ2=19.843, P<0.001 [Kruskal–Wallis test]). The bivariate correlation analysis was used (rs=0.141, P=0.000 [two-sided, Pearson]; rs=0.153, P=0.000 [two-sided, Spearman]), and our data showed that the results of two tests were similar. We found that patients with hyperplastic lesions had a worse effect with PDL.
Association of the PDL effect with the number of treatments
The greatest number of treatments among the 848 PWS patients was 20, while the least number was three. We divided the treatment times of these patients into two groups. Among the groups, the better effect of PDL treatment was the group of more than five times (305/422), with a RR of 72.3% and cure rate of 7.6%. The worst effect was in the group who had three to five treatments (287/426), with the RR of 67.3% and cure rate of 4.9% (Table 7).
There was a correlation between the number of treatments and the response to PWS (χ2=8.002, P=0.005 [Kruskal–Wallis test]). A bivariate correlation analysis was executed (rs=0.101, P=0.003 [two-sided, Pearson]; rs=0.097, P=0.005 [two-sided, Spearman]), and we found that these two tests were corresponding. There is an association of the PDL effect with the number of treatments, and the patients who were treated more times had the better response.
Discussion
We performed a large retrospective study of 848 cases to investigate the efficacy of PDL and related factors in the treatment of PWS in Chinese patients with skin type III–IV. There was a relatively high effect of PDL (RR =69.9% [592/848]) but a low complete clearance (cure rate of 6.3% [53/848]) in Chinese patients with PWS. In our study, the effect of PDL was associated with five factors of PWS patients, including age at treatment, location, and size of lesions, presence of existing hyperplastic lesions, and the number of treatments.
The results obtained in this study were close to the previous study of Ho et al in 2002.7 In their study, over 60% of patients had more than 25% clearing, and the majority of patients (41.1%) had 25%–50% of clearing. Less than one-quarter of patients (23%) experienced more than 50% clearing, and no patient had complete clearing. The results of our study confirmed the conclusion of Laube et al in 2003 who found that that the 595 nm PDL appeared to achieve further lightening of therapy-resistant PWSs in the majority of patients (67%).8 However, the treatment of PWS in the study of Ho et al used 585 nm PDL. There was no statistically significant difference between total effect of 595 nm PDL and 585 nm PDL, but the cure rate with 595 nm PDL was higher than with 585 nm PDL, and Chinese patients with PWS had higher incidence of pigmentation changes after receiving treatment of 585 nm PDL.11
We found that there was a relationship between the effects of PDL and the five characteristics of PWS patients. One of the factors was the age of patients at treatment, for which the results revealed that the younger patients had the better response. This may be related to the skins of younger patients, who have thinner dermis, less epidermal melanin, and less dermal collagen, which could reduce the light backscattered out of the skin and lower the fractional blood volume.12 Therefore, the timing of PDL treatment depended on the ages – this viewpoint conformed to a previous study that suggested that high-energy PDL had favorable effective in infants ≤6 months with facial PWS.13 Although the results provided new information for clinicians for choosing better treatment for patients with PWS, the number of patients older than 50 years was too small, which was the major limitation of the study. Past research indicated the age of PWS patients was not important for treatment timin,14 and one article showed a better effect of PDL in children with PWS was also associated with other factors.15
Another factor of affecting the treatment was the areas of lesions in PWS patients. There was a study that indicated centrofacial lesions in patients with PWS had lower response than lesions elsewhere on the head and neck,16 and this may result from the variable skin thickness of different areas. Our study showed that the effects on facial lesions were better than on neck, trunk, and extremities, which was the same as in previous studies.17,18 And we further confirmed that effects on lesions in different facial areas were variable and that the treatment effects in the temporal region were the best. Then another factor affecting PDL treatment was the sizes of skin lesions of PWS patients. The smaller size the lesion, the better was the efficacy, which proved the size of skin lesions was a factor affecting treatment.15 The presence of existing hyperplastic lesions of PWS patients was another factor. The skin lesions of two-thirds of PWS patients usually change to hyperplastic after 40 years, according to a previous study.4 Effects on the patients without hyperplastic lesions were better than on the patients with hyperplastic lesions who have the thicker skin; the reason may be optical shielding of overlying superficial vessels, and the fact that the penetration depth of light emitted by PDL was limited.19 An earlier research revealed that all of three PWS patients with hypertrophic lesions achieved significant lightening and that 12 of 17 patients who had the PDL-resistant lesions obtained moderate lightening after receiving a 755 nm alexandrite laser treatment.20 The 755 nm alexandrite laser has been reported to be particularly effective for hypertrophic or nodular lesions and PDL-resistant PWS, which may show a relationship with deeper blood vessels.5 The treatment effect of hyperplastic lesions with PDL has not been good, so some studies have investigated the curative effect of long-pulsed neodymium-doped yttrium aluminum garnet (Nd:YAG) laser.19,21 One study proved that hypertrophic PWS responds favorably to 1,064 nm laser therapy.19
The number of treatments was also one of the factors affecting PDL treatment, with the patients with greater number of treatments having the better response. The results showed that patients who received a larger number of treatments would have the better response. This is the same viewpoint as in a previous study.15
Although we did not analyze the relationship between different parameters of 595 nm PDL laser and the effect on PWS, we thought there might be an influence. For PWS patients, 595 nm PDL was a safe treatment method with more clearance and less adverse reaction.22 Aimed at the treatment of individual situation in patients, the physician must adjust the parameters with 595nm PDL flexibly. There were also some studies that showed that the settings of 595 nm PDL parameters were very important.23 Variation of PDL wavelength and/or pulse duration has been recommended to reach smaller and deeper vessels.24,25
During the last three decades, some laser systems and other adjuvant treatment therapies for PWS have been developed. Laser speckle imaging has been used to evaluate the degree of photocoagulation in laser therapy, which is essential for clinicians in deciding the necessity of retreatment of specific regions.26 And plethysmography could be developed to assess the features of vascular skin lesions such as PWSs.27 Research has suggested that suction devices can make the endothelial cells release NO, which in turn, induces hemangiectasis.26 In addition, it has been reported that the treatment effectiveness of photodynamic therapy was equal to or even better than PDL therapy.5 Some studies have supplied important evidence on novel treatment therapies for PWS patients, for instance combining PDL with rapamycin or combining selective photothermolysis with prothrombotic and/or antifibrinolytic medication regimen.28,29
The data of our research indicated that the PDL had a high RR but a low clearance rate in Chinese PWS patients; however, the efficacy assessment, which was based on the evaluation of only one dermatologist, had limitations. As well, the earlier the interventions, the better was the efficacy. The effect of PDL was also related to the anatomical area, the lesion size, presence of existing hyperplastic lesions, and number of treatment – this has important significance for clinical work.
Acknowledgments
We thank the individuals who participated in this project. This work was funded by a grant from the Natural Science Foundation of China (grant number 81271773) and Shandong Province Science and Technology Projects (grant number 2012GSF11835).
Disclosure
The authors report no conflicts of interest in this work.
References
Smoller BR, Rosen S. Port wine stains. A disease of altered neural modulation of blood vessels? Arch Dermatol. 1986;122(2):177–179. | ||
Waner M, Suen JY, editors. Hemangiomas and Vascular Malformations of the Head and Neck. New York, NY: John Wiley & Sons; 1999. | ||
Cordoro KM, Speetzen LS, Koerper MA, Frieden IJ. Physiologic changes in vascular birthmarks during early infancy: Mechanisms and clinical implications. J Am Acad Dermatol. 2009;60(4):669–675. | ||
van Drooge AM, Beek JF, van der Veen JP, van der Horst CM, Wolkerstorfer A. Hypertrophy in port wine stains: prevalence and patient characteristics in a large patient cohort. J Am Acad Dermatol. 2012;67(6):1214–1219. | ||
Chen JK, Ghasri P, Aguilar G, et al. An overview of clinical and experimental treatment modalities for port wine stains. J Am Acad Dermatol. 2012;67(2):289–304. | ||
Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220(4596):524–527. | ||
Ho WS, Chan HH, Ying SY, Chan PC. Laser treatment of congenital facial port wine stains: long-term efficacy and complication in Chinese patients. Lasers Surg Med. 2002;30(1):44–47. | ||
Laube S, Taibjee S, Lanigan SW. Treatment of resistant port wine stains with the V Beam pulsed dye laser. Lasers Surg Med. 2003;33(5):282–287.\ | ||
Currie CL, Monk BE. Can the response of port-wine stains to laser treatment be reliably assessed using subjective methods? Br J Dermatol. 2000;143(2):360–364. | ||
Ravnbak MH. Objective determination of Fitzpatrick skin type. Dan Med Bull. 2010;57(8):B4153. | ||
Chang CJ, Kelly KM, Van Gemert MJ, Nelson JS. Comparing the effectiveness of 585-nm vs 595-nm wavelength pulsed dye laser treatment of port wine stains in conjunction with cryogen spray cooling. Lasers Surg Med. 2002;31(5):352–358. | ||
Ortiz AE, Nelson JS. Port wine stain laser treatments and novel approaches. Facial Plast Surg. 2012;28(6):611–620. | ||
Chapas AM, Eickhorst K, Geronemus RG. Efficacy of early treatment of facial port wine stains in newborns: a review of 49 cases. Lasers Surg Med. 2007;39(7):563–568. | ||
van der Horst CM, Koster PH, de Borgie CA, Bossuyt PM, van Gemert MJ. Effect of the timing of treatment of port wine stains with the flash-lamp-pumped pulsed-dye laser. N Engl J Med. 1998;338(15):1028–1033. | ||
Nguyen CM, Yohn JJ, Huff C, Weston WL, Morelli JG. Facial port wine stains in childhood: prediction of the rate of improvement as a function of the age of the patient, size and location of the port wine stain and the number of treatments with the pulsed dye (585 nm) laser. Br J Dermatol. 1998;138(5):821–825. | ||
Renfro L, Geronemus RG. Anatomical differences of port wine stains in response to treatment with the pulsed dye laser. Arch Dermatol. 1993;129(2):182–188. | ||
Sommer S, Seukeran DC, Sheehan-Dare RA. Efficacy of pulsed dye laser treatment of port wine stain malformations of the lower limb. Br J Dermatol. 2003;149(4):770–775. | ||
Woo SH, Ahn HH, Kim SN, Kye YC. Treatment of vascular skin lesions with the variable-pulse 595 nm pulsed dye laser. Dermatol Surg. 2006;32(1):41–48. | ||
van Drooge AM, Bosveld B, van der Veen JP, de Rie MA, Wolkerstorfer A. Long-pulsed 1,064 nm Nd:YAG laser improves hypertrophic port wine stains. J Eur Acad Dermatol Venereol. 2013;27(11):1381–1386. | ||
Izikson L, Nelson JS, Anderson RR. Treatment of hypertrophic and resistant port wine stains with a 755 nm laser: a case series of 20 patients. Lasers Surg Med. 2009;41(6):427–432. | ||
Yang MU, Yaroslavsky AN, Farinelli WA, et al. Long-pulsed neodymium:yttrium-aluminum-garnet laser treatment for port wine stains. J Am Acad Dermatol. 2005;52(3 Pt 1):480–490. | ||
Faurschou A, Olesen AB, Leonardi-Bee J, Haedersdal M. Lasers or light sources for treating port wine stains. Cochrane Database Syst Rev. 2011;11:CD007152. | ||
Sivarajan V, Maclaren WM, Mackay IR. The effect of varying pulse duration, wavelength, spot size, and fluence on the response of previously treated capillary vascular malformations to pulsed-dye laser treatment. Ann Plast Surg. 2006;57(1):25–32. | ||
Jasim ZF, Handley JM. Treatment of pulsed dye laser-resistant port wine stain birthmarks. J Am Acad Dermatol. 2007;57(4):677–682. | ||
Rajaratnam R, Laughlin SA, Dudley D. Pulsed dye laser double-pass treatment of patients with resistant capillary malformations. Lasers Med Sci. 2011;26(4):487–492. | ||
Aguilar G, Choi B, Broekgaarden M, et al. An overview of three promising mechanical, optical, and biochemical engineering approaches to improve selective photothermolysis of refractory port wine stains. Ann Biomed Eng. 2012;40(2):486–506. | ||
Verkruysse W, Svaasand LO, Nelson JS. Remote plethysmographic imaging using ambient light. Opt Express. 2008;16(26):21434–21445. | ||
Tan W, Jia W, Sun V, Mihm MC, Nelson JS. Topical rapamycin suppresses the angiogenesis pathways induced by pulsed dye laser: molecular mechanisms of inhibition of regeneration and revascularization of photocoagulated cutaneous blood vessels. Lasers Surg Med. 2012;44(10):796–804. | ||
Heger M, Beek JF, Moldovan NI, van der Horst CM, van Gemert MJ. Towards optimization of selective photothermolysis: prothrombotic pharmaceutical agents as potential adjuvants in laser treatment of port wine stains. A theoretical study. Thromb Haemost. 2005;93(2):242–256. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.