Back to Journals » Research and Reports in Urology » Volume 7
Transurethral convective water vapor as a treatment for lower urinary tract symptomatology due to benign prostatic hyperplasia using the Rezūm® system: evaluation of acute ablative capabilities in the human prostate
Authors Dixon C, Cedano ER, Mynderse L, Larson TR
Received 10 September 2014
Accepted for publication 9 October 2014
Published 30 January 2015 Volume 2015:7 Pages 13—18
DOI https://doi.org/10.2147/RRU.S74040
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Christopher M Dixon,1 Edwin Rijo Cedano,2 Lance A Mynderse,3 Thayne R Larson4
1Lenox Hill Hospital, New York, NY, USA; 2Department of Urology, Clinica Canela, La Romana, Dominican Republic; 3Department of Urology, Mayo Clinic, Rochester, MN, USA; 4Institute of Medical Research, Scottsdale, AZ, USA
Background: The purpose of this study was to assess the acute ablative characteristics of transurethral convective water vapor (steam) using the Rezūm® system in men with benign prostatic hyperplasia through histologic and radiographic studies.
Methods: Seven patients were treated with transurethral intraprostatic injections of sterile steam under endoscopic visualization followed by previously scheduled adenectomies. The extirpated adenomas were grossly examined followed by whole mount sectioning and staining with triphenyl-tetrazolium chloride (TTC) to evaluate thermal ablation. Histology was performed after hematoxylin and eosin staining on one prostate. After review of results from the first patient cohort, an additional 15 patients with clinical benign prostatic hyperplasia were treated followed by gadolinium-enhanced magnetic resonance imaging (MRI) at one week.
Results: In the first patient cohort, gross examination of TTC-stained tissue showed thermal ablation in the transition zone. In addition, there was a distinct interface between viable and necrotic prostatic parenchyma. Histopathologic examination revealed TTC staining-outlined necrotic versus viable tissue. Gadolinium-enhanced MRIs in the cohort of 15 patients demonstrated lesion defects in all patients at 1 week post-procedure. Coalesced lesions were noted with a mean (± standard deviation) lesion volume of 9.6±8.5 cm3. The largest lesion volume was 35.1 cm3. Ablation using vapor was rapid and remained confined to the transition zone, consistent with the thermodynamic principles of convective thermal energy transfer.
Conclusion: Thermal ablation was observed in all specimens. The resulting coalescing ablative lesions, as seen on MRI, were confined to the transition zone. These studies confirm the ablative capabilities of vapor, validate the thermodynamic principles of convective heating, and allow for further clinical studies.
Keywords: steam, vapor therapy, thermotherapy, minimally invasive, lower urinary tract symptomatology, benign prostatic hyperplasia
Introduction
The Rezūm® system is a minimally invasive treatment for lower urinary tract symptomatology utilizing the unique properties of convective energy transfer as described by the laws of thermodynamics. This contrasts with the conductive characteristics of other minimally invasive therapies such as transurethral microwave and radiofrequency modalities. Convection is the movement of a heated gas or liquid within a defined space. Steam (vapor) fills a defined space between the cells and transfers energy onto the exterior of cell membranes. Conduction is the transfer of heat through a nonmoving material from the area of higher temperature to lower temperature.1
The Rezūm system, via a transurethral approach, injects water vapor into the transition zone of the prostate at approximately 103°C. The vapor disperses through the tissue interstices using infusion pressures slightly above interstitial pressure and convectively delivers thermal energy, while staying contained within the transition zone.
When heated to steam, water expands approximately 1,700 times its liquid volume and carries up to 540 calories of energy per gram. This stored thermal energy is released when steam phase shifts or condenses from vapor to liquid upon contact with tissue. The temperature of tissue treated with vapor in the 9-second treatment has been demonstrated to reach between 70°C and 80°C,2 consistent with the temperature range at which cell death occurs instantaneously.3
The objective of the early studies reported herein was to determine whether convective thermal energy transfer resulting from vapor delivery by the Rezūm system was capable of rapid and effective ablation of human prostate tissue. To validate the principles of convective steam delivery to treat lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and assess the ablative characteristics, a series of histological and radiographic studies were performed in two initial studies. In the first study, a histological vital stain was used on extirpated prostate tissue to demarcate viable and necrotic tissue previously treated with the Rezūm system. In the second study, gadolinium-enhanced magnetic resonance imaging (MRI) was performed 1 week following treatment with the Rezūm system to visualize the ablative effect and to evaluate location of the resultant lesions.
Materials and methods
All human studies were approved by the appropriate ethics committee and were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects gave their informed consent prior to their inclusion in the study.
Under ethics committee approval from a single treatment center, 22 men were recruited from a cohort of patients with symptomatic BPH. Patients were excluded if they had a penile prosthesis, any prior minimally invasive BPH procedure, confirmed or suspected prostate carcinoma, prior radiation therapy, urethral strictures, or a biopsy of the prostate within 30 days, or were unable or unwilling to provide informed consent.
The Rezūm system generates vapor by application of radiofrequency energy to create heat via electromagnetic induction in the handle of the delivery device and incorporates a standard endoscopic cystoscopy lens to visualize the positioning of the treatment needle into the obstructing BPH tissue. A PEEK (polyether ether ketone) needle is smoothly and rapidly deployed under direct visualization into the obstructive regions of the transition zone and the steam is delivered into the tissue from an array of vapor emitter holes spaced 120 degrees circumferentially around the tip of the needle. Each injection of steam is for approximately 9 seconds. The number of injections in each prostate is determined by the size of the adenoma and length of the prostatic urethra.
In the first study, seven patients scheduled for surgical removal of prostate tissue were treated with transurethral steam therapy under the same anesthetic as for the planned open adenectomy (Table 1). The number of steam injections was determined by prostatic urethral length and presence/absence of a median lobe. Following treatment with vapor, adenectomies were performed and the fresh, extirpated adenomas were grossly examined followed by immediate whole mount sectioning and preparation for and staining with triphenyl-tetrazolium chloride (TTC). TTC stains viable tissue with healthy metabolic enzymes as a bright red color, while irreversibly damaged tissue with no metabolic enzymes does not take up stain and remains its natural tan color.4
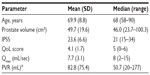
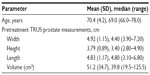  | Table 1 Baseline patient characteristics for the first patient cohort (n=7) |
Selected areas from the TTC-stained fresh whole mounts of one prostate were then dissected and preserved in 10% formalin for standard hematoxylin and eosin (H&E) staining. Histological analysis was used to demarcate viable and necrotic tissue as well as areas of interest such as the urothelium and thermal boundaries.
After analyzing extirpated tissues from the first seven patients, another ethics committee protocol was approved for a second cohort of 15 patients (Table 2). The only relevant difference in inclusion/exclusion criteria between the two patient cohorts was that patients in the second group were to have their BPH treated with the Rezūm system without undergoing adenectomy. While there were other standard exclusion criteria unique to the second cohort (eg, concomitant use of medications, bladder or sphincter abnormalities), these additional criteria were related to the long-term clinical follow-up of these patients, which is not reported herein. Gadolinium-enhanced MRI was performed on each subject 1 week posttreatment to assess volume and location of the ablative lesions. The MRIs were analyzed by the Mayo Clinic Biomedical Image Resource using Analyze 10.1™ software, and three-dimensional volumetric renderings were created to measure the prostate, transition zone, and areas of treatment as indicated by the gadolinium defect.
Results
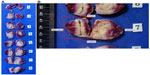
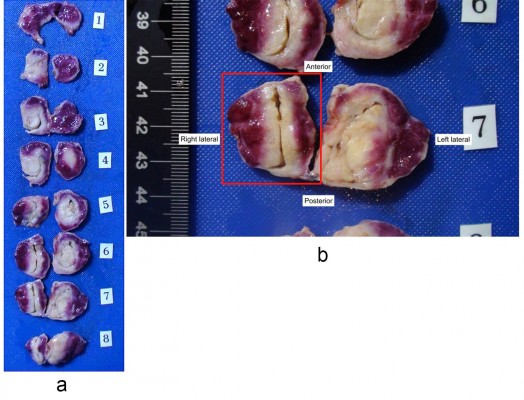
In the adenectomy group, the mean number of steam injections per lateral lobe was 4.1 (range 3–6), and the median lobe was treated in two patients (Table 3). There were no thermal effects seen outside of the prostate at the time of adenectomy on gross examination. Each extirpated adenoma showed defects identified by vital staining that correlated with the injection sites. The treatments coalesced and produced lesions in the transition zones visible via vital staining. Lesions were observed in the tissue and the tissue was sectioned axially (Figure 1A). The largest lesions from each adenoma averaged 18.0 mm ×18.9 mm, with the largest lesion being 21 mm ×28 mm (Figure 1B).
  | Table 3 Treatment parameters |
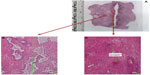
H&E histologic staining of the one excised adenoma tissue demonstrated distinct demarcations between nonviable treated tissue and untreated prostate. This correlated well with TTC whole mount specimens (Figure 2A and B). H&E also demonstrated thrombosed vasculature (Figure 2C) and general preservation of the prostatic urethral epithelium (Figure 3A and B). There was no carbonization or heat fixation observed in the excised tissue.
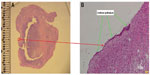  | Figure 3 (A) Whole mount hematoxylin and eosin section of the left lobe. (B) Preservation of urethral epithelium at 10× magnification. |
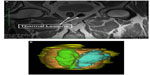
In the Rezūm system-treated group without adenectomy, the mean number of steam injections per lateral lobe was 2.3 (range 1–4) and the mean number of injections per median lobe in four patients was 2.5 (range 2–3; Table 3). The MRIs of these 15 treated patients demonstrated prostatic gadolinium defects in all patients at 1 week from therapy. This merging of treatment zones appeared to mimic that which was observed using TTC staining on adenectomy whole mount specimens. Coalesced lesions were noted in each lateral lobe corresponding to the targeted treatment sites. At 1 week, the mean (± standard deviation) gadolinium defect volume was 9.6±8.5 cm3 (Table 4). The largest defect volume was 35.1 cm3 (Figure 4A and B). Gadolinium defects have been shown to correlate with histological proven necrosis in previous studies.5 Defects were observed in the transition zone. No thermal effects were seen in the peripheral zone. In several cases, the gadolinium defect outlined the boundary of the transition zone without passing through to the peripheral zone (Figure 5A and B).
  | Table 4 Lesion characteristics |
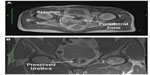  | Figure 5 Gadolinium-enhanced transverse (A) and coronal (B) magnetic resonance images showing large thermal lesions outlining the transition zone while preserving the peripheral zone and urethra. |
Discussion
By observing both TTC-stained whole mounts and MRIs of the treated tissues, we conclude that 9-second treatments of transurethral convective heating with water vapor can rapidly ablate human prostate tissue in the transition zone. Because of the convective thermal transfer using steam, lesions were observed within the McNeal zones of the prostate where the steam had been injected.6 These properties cause steam to travel through the interstices between cells until it encounters boundaries. At the boundary, such as the tissue planes between prostatic zones, steam will follow the perimeter similar to the way wind blows against a sail without penetrating. These unique properties are further supported by MRIs of the 15 subjects demonstrating defects outlining the transition zone and hyperplastic tissue without extension into the peripheral zone.
In conclusion, significant ablation of prostatic tissue is possible with convective steam treatments of approximately 9 seconds per injection. Further studies are warranted to confirm the clinical benefit and safety of prostatic ablation with steam.
Author contributions
CMD, project development, data collection, manuscript writing; ERC, data collection, manuscript writing; TRL, project development, data collection, and manuscript writing; LAM, data collection and manuscript writing. All authors approved the final version and agree to be accountable for all aspects of the work.
Disclosure
CMD and TRL are consultants for NxThera, Inc. ERC and LAM have no conflicts of interest to report. This work was supported clinically and financially by NxThera Inc.
References
Hahn DW and özişik MN. Heat Conduction Fundamentals, in Heat Conduction. Third Edition. John Wiley & Sons, Inc., Hoboken, NJ, USA. 2012. | |
Data on file. NxThera, Maple Grove, MN, USA. | |
Bhowmick P, Coad JE, Bhowmick S, et al. In vitro assessment of the efficacy of thermal therapy in human benign prostatic hyperplasia. Int J Hyperthermia. 2004;20:421–439. | |
Fishbein MC, Meerbaum S, Rit J, et al. Early phase acute myocardial infarct size quantification: Validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593–600. | |
Larson BT, Collins JM, Huidobro C, et al. Gadolinium-enhanced MRI in the evaluation of minimally invasive treatments of the prostate: correlation with histopathologic findings. Urology. 2003;62:900–904. | |
McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2: 35–49. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.