Back to Journals » Clinical Ophthalmology » Volume 9
Topical difluprednate for the treatment of Harada’s disease
Authors Onishi S, Asahi M , Chou C, Gallemore R
Received 20 August 2014
Accepted for publication 16 October 2014
Published 21 January 2015 Volume 2015:9 Pages 157—167
DOI https://doi.org/10.2147/OPTH.S72955
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Spencer M Onishi, Masumi G Asahi, Calvin Chou, Ron P Gallemore
Retina Macula Institute, Torrance, CA, USA
Purpose: To describe the use of topical difluprednate for treatment of patients who presented with Harada’s disease.
Methods: Retrospective case series of patients managed with topical difluprednate alone at the onset of the diagnosis. The patients were followed with optical coherence tomography (OCT) and fluorescein angiography.
Results: In our three cases, complete resolution of the exudative detachments with improvement in visual acuity was achieved in each case. Central macular thickness was reduced by a mean of 365±222 µm. Initial loading dose was one drop every hour while awake, followed by a variable tapering regimen. Leakage on fluorescein angiography and exudative detachments on OCT both responded to treatment with difluprednate. In two of the three cases, the patients recovered vision to 20/20, and the third case recovered to 20/25. Steroid-induced glaucoma was observed and managed with one to two glaucoma drops as needed.
Conclusion: Difluprednate is effective for managing ocular manifestations of Harada’s disease. Further studies of this drug for the management of noninfectious posterior uveitis are warranted.
Keywords: serous retinal detachment, noninfectious posterior uveitis, steroid
Introduction
Vogt–Koyanagi–Harada disease (VKH) is a multisystem disorder with ocular manifestations including granulomatous panuveitis.1 Cutaneous manifestations include poliosis, alopecia, and vitiligo of the eyelashes and eyebrows. Affected patients are typically 20–50 years of age and are most commonly Asian, Middle Eastern, Hispanic, or Native American.1 VKH characteristically presents with bilateral intraocular inflammation associated with serous retinal detachments and hyperemia of the optic disc. Symptoms typically occur bilaterally; however, one eye may deteriorate 2–3 weeks before the contralateral eye.2 The term Harada’s disease has been used for VKH patients with primarily posterior uveitis.
Without prompt medical intervention, Harada’s disease carries a poor visual prognosis. Traditional treatment options focus on the acute use of systemic immunosuppressive therapy, including high-dose prednisone (2 mg/kg induction) followed by steroid-sparing immunosuppressive agents as the course of the disease requires.1 These agents are associated with potentially serious side effects as a prolonged slow tapering course of treatment is typically required to reduce recurrence. In the present study, we report three cases of Harada’s disease effectively managed topically with the strong steroid difluprednate (Durezol, Alcon Laboratories, Inc, Fort Worth, TX, USA), without the use of more invasive therapy such as subtenon kenalog, intravitreal triamcinolone, fluocinolone implantation, or oral systemic therapy. This novel molecule, which is approved for the treatment of endogenous anterior uveitis, has recently been shown to be noninferior to prednisolone acetate 1%.3
Methods
A retrospective chart review was performed on three patients who presented to the Retina Macula Institute (Torrance, CA, USA) with a history of unilateral or bilateral unexplained progressive vision loss. VKH was diagnosed on the basis of clinical exam and fluorescein angiography (FA) findings. Response to treatment was also monitored with macular optical coherence tomography (OCT) measurements using spectral domain optical coherence tomography (SD-OCT) on the Cirrus HD-OCT (Carl Zeiss Meditec AG, Jena, Germany).
Case 1
A 54-year-old Hispanic female presented with decreasing distance vision OD (oculus dexter, right eye) as well as difficulty reading. Visual acuity (VA) measured 20/40 OD, 20/20 OS (oculus sinister, left eye) with an intraocular pressure (IOP) of 12 mmHg OD and 11 mmHg OS. Review of systems was negative and the patient reported a family history of cataracts, cancer, diabetes, and hypertension.
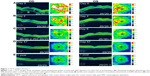
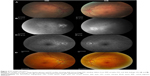
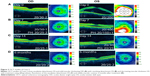
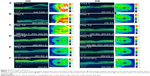
Dilated funduscopic exam revealed 3+ cells in the anterior chamber (AC) and vitreous cavity and a serous detachment in the macula OD. OCT studies confirmed the presence of subretinal fluid (SRF) and revealed irregular elevations of the retinal pigment epithelium (RPE) OU (oculus uterque, both eyes) (Figure 1A). FA showed a disk hemorrhage OD with leakage from the macula and optic disc OU (Figure 2A and B). No perfusion defects were present, arguing against a vasculitis process (Figure 2B). An aggressive course of topical difluprednate 0.05% was prescribed with an initial loading dose of one drop every hour (q1h) OU. The patient underwent a workup and was found to be positive for HLA-DRB1 (DR4).4 Three days later, the VA improved to 20/25+ OD, 20/20-3 OS. IOP remained stable at 11 OD and 13 OS. OCT studies showed gradual resolution of the SRF and RPE elevations, while FA studies showed reduced leakage (Figures 1B, 1C, and 2C). Difluprednate was tapered to QID for 1 week, TID for 1 week, BID for 1 week, qd (once daily) for 1 week, then discontinued. After this initial 4-week course of treatment, normal macular contour was maintained, and the patient was switched to topical loteprednol BID for 6 months (Figures 1D and 2D). A year after her initial presentation, OCT studies showed absence of SRF in both eyes (Figure 1E). In the subsequent year, she had three bouts of recurrent inflammation, which were each managed with hourly topical difluprednate for 1–2 days, then a similar taper as mentioned above, and continuation of the loteprednol BID. At 4-year follow-up, she remained 20/20− OD, 20/25-1 OS, IOP of 14 OD and 11 OS, with trace posterior subcapsular cataract (PSC) OU off medication.
Case 2
A 40-year-old Middle Eastern female presented with progressive blurriness (OS>OD) associated with central photopsias. She also noted mild headaches with binocular diplopia. VA measured 20/30-2 OD, 20/200 OS with IOP of 19 OD and 20 OS. Review of systems was negative, and she denied any prior ocular or family history.
Dilated funduscopic exam revealed trace white blood cells in the anterior vitreous and bilateral serous macular detachments. OCT studies confirmed the serous macular detachment OS with cystoid macular edema (CME) OU (Figure 3A). FA studies revealed punctate leakage in the macula and disk leakage OU late in the study (Figure 4A and B). Topical difluprednate was prescribed with an initial loading dose of one drop q1h OU while awake, and by day 7, VA improved to 20/40 OD and 20/80 OS with resolution of her headaches. IOP was noted to be 22 OD and 20 OS. Given the mild steroid response, the patient was started on dorzolamide 2%/timolol 0.5% once daily (qd) OU. OCT and FA studies showed a marked improvement with decreased SRF OS and reduced leakage, respectively (Figures 3B and 4C). By day 14, the patient recovered to 20/25-1 OD and 20/30 OS with pinhole to 20/25 OS. OCT studies showed resolution of the serous detachment OS and decreased activity on FA studies (Figures 3C and 4D). Given the improvement, difluprednate was tapered to q3h for 1 week, QID for 1 week, TID for 1 week, BID for 2 weeks, and qd thereafter for 2 months. The patient ultimately recovered to 20/20-2 OD and 20/30-2 OS with IOP of 21 OU. Given the mild residual CME OS, the patient continued difluprednate qd OU in addition to bromfenac 0.07% qd OS for 3 months. It was also necessary to increase dorzolamide 2%/timolol 0.5% to BID OU for IOP control. At 6 months, she was reevaluated clinically and with imaging, which showed that her lenses remained clear and vision remained stable at 20/20− OD and 20/25− OS with IOP of 18 OD and 20 OS while continuing management with difluprednate 0.05% qd OU, bromfenac 0.07% qd OS, and dorzolamide 2%/timolol 0.5% BID OU (Figures 3D and 4E).
Case 3
A 48-year-old Hispanic female with a history of VKH syndrome presented to our clinic with progressive vision loss OD associated with floaters for one month. Corrected VA was 20/100 OD and was 20/20− OS. Dilated funduscopic exam revealed 1+ cells in the AC and vitreous cavity, and a serous detachment with RPE elevation in the macula OD, which was confirmed by OCT studies (Figure 5A). FA studies showed leakage in the macula and optic disc as well as punctate focal hyperfluorescent spots late in the study OU (Figure 6A and B). Topical difluprednate was prescribed with an initial loading dose of one drop q1h OU while awake for 2 weeks with brimonidine 0.2%/timolol 0.5% BID in anticipation of a steroid response. On day 3, there was a marked decrease in SRF OD on OCT studies (Figure 5B). On day 10, VA improved to 20/60 OD and 20/25 OS with continued resolution of the SRF OD (Figure 5C). IOP remained stable at 15 OU, despite the aggressive course of topical steroids. FA studies were again performed, which showed persistent leakage from the macula and optic disc late in the study OU (Figure 6C). The patient was instructed to continue her drops until her next visit. On day 18, her vision improved to 20/30+2 OD, 20/40 OS, PH to 20/20 OS with resolution of the RPE elevation with mild residual SRF (Figure 5D), so the difluprednate was reduced to every 2 hours while awake. On day 31, OCT studies showed resolving displacement of the exudative detachment, but she now presented with increasing severity of headaches (Figure 5E). VA was maintained at 20/20-2 OD, 20/25-1 OS, but because she now presented with systemic involvement, the patient continued her drops and started a course of methylprednisolone dose pack (4 mg), after which she started prednisone 20 mg daily for 2 weeks, 15 mg daily for 2 weeks, 10 mg daily for one month, and began methotrexate 10 mg per week for 10 weeks as instructed by her rheumatologist. At the 3-month follow-up, difluprednate was reduced to QID. Two months later, the patient was continuing to improve, but she spiked an IOP of 18 OD and 30 OS. Difluprednate was then discontinued, and the patient was placed on dorzolamide 2%/timolol 0.5% TID, brimonidine TID, and prednisolone acetate 1% BID. At her 6-month follow-up, the macular contour remained stable in both eyes, and her vision improved to 20/20 OD and 20/20 OS, and IOP recovered to 15 OD, 14 OS (Figures 5F, 6D, and 6E).
Results and discussion
The standard of care for initial therapy for VKH is an aggressive course of oral corticosteroids with prednisone equivalent of 1–2 mg/kg/d.1 Increased frequency of the HLA alleles in patients with VKH suggests a possible autoimmune pathogenesis.4,5 As with other forms of uveitis, eicosanoids appear to play a primary role in the acute phase inflammatory response of Harada’s disease where the interleukin pathway mediates longer-term effects, both of which are suppressed by steroids.6,7 Topical steroid drops are utilized to treat anterior segment inflammation, while intravenous methylprednisolone therapy is reserved for cases in which retinal detachments do not respond to both oral and topical steroids.1 Cunha et al have also reported treatment of VKH with posterior Subtenon steroid injections as adjunctive therapy.8
Our three cases demonstrate the resolution of serous detachments and AC inflammation with topical difluprednate alone. Macular edema (ME) was profoundly reduced by a mean of 365±222 μm, while vision was improved on the therapy. Difluprednate is a “strong” topical steroid now approved for use in anterior uveitis as well as postoperative inflammation and pain. Animal studies and limited human clinical trials suggest that difluprednate can penetrate the posterior segment,9 where a single drop of difluprednate produced detectable levels in the choroid ranging from 59–273 ng eq/g (59 ng eq/g in the posterior retina/choroid and 273 ng eq/g in the anterior retina/choroid).10 There is data demonstrating absorption of difluprednate into the posterior segment including the retinal and RPE layers that may provide a reservoir of drug once the loading dose has been administered.11,12 We feel the hourly loading dose for 2 days or longer is critical in the management of these cases. Clinical studies in humans found that difluprednate was comparable to a posterior subtenon triamcinolone injection in the treatment of diabetic macular edema (DME) in addition to showing superiority over topical betamethasone for DME.11 Of note, a prolonged, slow tapering course of systemic steroids is often required to treat Harada’s disease. We likely tapered too quickly in Case 1, accounting for the rebound responses, and used more prolonged tapering courses, often up to 6 months. In subsequent cases, frequent OCT studies monitoring for SRF and ME as well as IOP checks while on difluprednate should be performed and the medication tapered accordingly (every 2–4 weeks).
Difluprednate has a greater affinity than prednisolone for glucocorticoid receptors, resulting in increased tissue penetration and increased bioavailability, which accounts for its increased potency.12 It is also a prodrug with rapid corneal penetration and is delivered as an emulsion, potentially improving the access of drug compared with the suspension formulation of prednisolone acetate.10 The increased potency of the emulsion formula can help with compliance with treatment, as it does not have to be administered as often as prednisolone acetate.
Topical difluprednate has also been shown to have minimal systemic absorption, and its use can avoid the common complications associated with oral steroid use.10 Difluprednate is lipophilic with a partition coefficient of 3.40 and is absorbed through noncorneal absorption routes in addition to the transcorneal route.10 Additionally, ocular concentrations were at least 20 times greater than whole blood absorption.10 Ocular side effects may be increased, but with the prevention benefit of potentially serious systemic side effects associated with systemic therapy such as ulcers, hyperglycemia, bone resorption, adrenal insufficiency, or Cushing’s syndrome. Inappropriate administration of oral steroids or poor patient compliance can also lead to the aforementioned side effects. Given the high potency and comparable strength to a posterior subtenon triamcinolone injection, treatment with difluprednate should be considered a suitable treatment option for uveitic symptoms and serous detachments associated with VKH in the absence of neurological and cutaneous manifestations. For those cases with systemic involvement, oral corticosteroid therapy should be initiated, as was discussed in case three, where a severe headache developed despite treatment with difluprednate. Where there are instances of progression of neurologic symptoms, further systemic workup should also be considered.
The high (hourly) frequency of the loading dose does raise concerns for potential steroid-induced glaucoma and cataract formation, which should be considered and discussed prior to starting patients on difluprednate. Studies have shown that only a small percentage of patients (3%–6%) developed increased IOP with difluprednate dosed BID or QID at 14 days.13 The high frequency and long duration of use required in such cases make steroid-induced glaucoma more likely. However, in our presented cases, management of elevated IOP was attained with pressure-lowering ophthalmic drops with no further surgical intervention required. We observed a mild steroid response in two cases, which were appropriately managed with dorzolamide 2%/timolol 0.5% and brimonidine. Nonetheless, close follow-up is warranted, even weekly, with prolonged use of a strong steroid.14,15 On the basis of our findings, further studies are warranted to assess the efficacy of difluprednate for the management of noninfectious posterior uveitis.
Conclusion
The standard of care for initial therapy for VKH is an aggressive course of oral corticosteroids. Our three cases demonstrate the resolution of ocular manifestations of VKH including serous detachments and AC inflammation with topical difluprednate alone. Topical difluprednate has been shown to have minimal systemic absorption, and its use can avoid the common complications associated with oral steroid use. Given the high potency and noninvasive nature of therapy, treatment with difluprednate should be considered a suitable treatment option for uveitic symptoms and serous detachments associated with VKH in the absence of neurological and cutaneous manifestations.
Disclosure
The authors report no conflicts of interest in this work.
References
Andreoli CM, Foster CS. Vogt-Koyanagi-Harada disease. Int Ophthalmol Clin. 2006;46(2):111–122. | ||
Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647–652. | ||
Sheppard JD, Toyos MM, Kempen JH, et al. Difluprednate 0.05% versus prednisolone acetate 1% for endogenous anterior uveitis: a phase III, multicenter, randomized study. Invest Ophthalmol Vis Sci. 2014;55(5):2993–3002. | ||
Weisz JM, Holland GN, Roer LN, et al. Association between Vogt-Koyanagi-Harada syndrome and HLA-DR1 and -DR4 in Hispanic patients living in Southern California. Ophthalmology. 1995;102(7):1012–1015. | ||
Davis JL, Mittal KK, Freidlin V, et al. HLA associations and ancestry in Vogt-Koyanagi-Harada disease and sympathetic ophthalmia. Ophthalmology. 1990;97(9):1137–1142. | ||
El-Asrar AM, Struyf S, Kangave D, et al. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Clin Immunol. 2011;139(2):177–184. | ||
Bucolo C, Cuzzocrea S, Mazzon E, et al. Effects of cloricromene, a coumarin derivative, on endotoxin-induced uveitis in Lewis rats. Invest Ophthalmol Vis Sci. 2003;44(3):1178–1184. | ||
Cunha R, Martins RG, Brito H, et al. Posterior subtenon triamcinalone acetonide injection as an adjunctive treatment in patients with recurrent Vogt-Koyanagi-Harada syndrome. Invest Ophthalmol Vis Sci. 2010;51:E-Abstract 5831. | ||
Yamaguchi M, Yasueda S, Isowaki A, et al. Formulation of an ophthalmic lipid emulsion containing an anti-inflammatory steroidal drug, difluprednate. Int J Pharm. 2005;301:121–128. | ||
Tajika T, Isowaki A, Sakaki H. Ocular distribution of difluprednate ophthalmic emulsion 0.05% in rabbits. J Ocul Pharmacol Ther. 2011;27(1):43–49. | ||
Nakano S, Yamamoto T, Kirii E, Abe S, Yamashita H. Steroid eye drop treatment (difluprednate ophthalmic emulsion) is effective in reducing refractory diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2010;248:805–810. | ||
Foster CS, DaVanzo R, Flynn TE, et al. Durezol (difluprednate ophthalmic emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J Ocul Pharmacol Ther. 2010;26(5):475–483. | ||
Donnenfeld ED. Difluprednate for the prevention of ocular inflammation post surgery: an update. Clin Ophthalmol. 2011;5:811–816. | ||
Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics: I, the effect of dexamethasone in the normal eye. Arch Ophthalmol. 1963;70:482–491. | ||
Jamal KN, Callanan DG. The role of difluprednate ophthalmic emulsion in clinical practice. Clin Ophthalmol. 2009;3:381–390. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.