Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
TNYL peptide functional chitosan-g-stearate conjugate micelles for tumor specific targeting
Authors Chen F, Yan J, Yi H, Hu F, Du Y, Yuan H , You J, Zhao M
Received 17 June 2014
Accepted for publication 24 July 2014
Published 26 September 2014 Volume 2014:9(1) Pages 4597—4608
DOI https://doi.org/10.2147/IJN.S69572
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Feng-Ying Chen,1 Jing-Jing Yan,1 Han-Xi Yi,2 Fu-Qiang Hu,2 Yong-Zhong Du,2 Hong Yuan,2 Jian You,2 Meng-Dan Zhao1
1Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, People’s Republic of China; 2College of Pharmaceutical Science, Zhejiang University, Hangzhou, People’s Republic of China
Abstract: Nowadays, a real challenge in cancer therapy is to design drug delivery systems that can achieve high concentrations of drugs at the target site for improved therapeutic effect with reduced side effects. In this research, we designed and synthesized a homing peptide-(TNYLFSPNGPIA, TNYL) modified chitosan-g-stearate (CS) polymer micelle (named T-CS) for targeting delivery. The peptide displayed specific binding affinity to EphB4 which is a member of the Eph family of receptor tyrosine protein kinases. The amphiphilic polymer T-CS can gather into micelles by themselves in an aqueous environment with a low critical micelle concentration value (91.2 µg/L) and nano-scaled size (82.1±2.8 nm). The drug encapsulation efficiency reached 86.43% after loading the hydrophobic drug doxorubicin (DOX). The cytotoxicity of T-CS/DOX against SKOV3 cells was enhanced by approximately 2.3-fold when compared with CS/DOX. The quantitative and qualitative analysis for cellular uptake indicated that TNYL modification can markedly increase cellular internalization in the EphB4-overexpressing SKOV3 cell line, especially with a short incubation time. It is interesting that relatively higher uptake of the T-CS/DOX micelles by SKOV3 cells (positive-EphB4) than A549 cells (negative-EphB4) was observed when the two cells were co-incubated. Furthermore, in vivo distribution experiment using a bilateral-tumor model showed that there was more fluorescence accumulation in the SKOV3 tumor than in the A549 tumor over the whole experiment. These results suggest that TNYL-modified CS micelles may be promising drug carriers as targeting therapy for the EphB4-overexpressing tumor.
Keywords: chitosan-g-stearate, polymeric micelles, TNYL, active targeting, antitumor activity
Introduction
Current chemotherapy of cancer is limited by the narrow therapeutic index of most anticancer drugs due to the lack of selectivity toward tumor cells.1 Successful cancer treatment requires a unique carrier system that can load a large amount of drugs and specifically target tumor cells.2 Numerous delivery vehicles such as polymeric micelles, dendrimers, liposomes, and polymer–drug conjugates have emerged for solid-tumor targeting.3–6 Among them, polymeric micelles, formed from an amphiphilic block copolymer, have gained great attention due to their inherent properties such as nano-ranged size, prolonged blood circulation because of their high solubility in water, and low toxicity in the human body.7,8
Polymeric micelles are capable of encapsulating poorly water-soluble or hydrophobic anticancer drugs owing to their special core–shell structures. High entrapment efficiency can be achieved by optimizing the formulation.9 The physicochemical parameters can be accommodated to allow drug-loaded micelles to achieve tumor passive transport via the leakage vasculature surrounding the tumors through enhancing permeability and retention effects.1,10 However, the passive transportation of drugs in the tumor tissues does not ensure successful therapy all the time because nanocarriers often fail in escaping from immune surveillance and cycling for a long period of time.11 Extensive work has been committed to active-targeting treatment, in which the nanoparticles are conveyed to the target tissues with specific antigen–antibody interactions.12 The tumor cells usually overexpress some specific substances such as antigens or receptors on their surfaces. The ligands targeted participating in cell endocytic transport are conducive to improving the curative effect and reducing potential damage to healthy tissue. They are most commonly used in active targeting as they provide more controllable pharmacokinetics and bioavailability features.13 EphB4 receptors, members of the Eph family of receptor tyrosine kinase, are overexpressed in a wide variety of tumor cell membranes including prostate, colon, breast, and ovarian cancers.14–16 The homing peptide TNYLFSPNGPIA (termed as TNYL) can be specifically recognized by EphB4, which is a particularly promising method for tumor-specific delivery.17
Chitosan and its derivatives have received more and more attention because of their unique physicochemical and biological properties. We have developed an amphiphilic-grafted copolymer, ie, chitosan-g-stearate (CS) for the delivery of antitumor drugs.18 The biocompatible CS can form self-assembling micelles in water, and exhibit high drug encapsulation efficiency and excellent cellular internalization. Here, to further improve the antitumor efficiency of CS micelles, the CS was modified by TNYL to design a novel tumor active-targeting drug delivery system. Doxorubicin (DOX) was used as a model antitumor drug. The physicochemical properties of cellular uptake behavior and cytotoxicity in vitro with SKOV3 cell lines, as well as in vivo distribution of this drug delivery system were investigated in detail.
Materials and methods
Materials
Chitosan oligosaccharide (Mw [Mw=450.0 KDa] =17.5 KDa, 95% deacetylated degree) was obtained by enzymatic degradation from chitosan (Mw =450.0 KDa). Stearic acid was purchased from Shanghai Chemical Reagent Co., Ltd., (Shanghai, People’s Republic of China). 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), 2,4,6-trinitrobenzene sulfonic acid (TNBS), fluorescein isothiocyanate (FITC), pyrene, mPEG2000 with amine group (NH2-PEG-NH2), and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich Co., (St Louis, MO, USA). TNYL (sequence: TNYLFSPNGPIA) was synthesized by Guangzhou Sinoasis Pharmaceuticals Inc., (Guangzhou, People’s Republic of China). Cis-aconitic anhydride (CA) was provided by Alfa Aesar Co., (Ward Hill, MA, USA). Doxorubicin hydrochlorate (DOX.HCl) was gifted from Hisun Pharmaceutical Co., Ltd, (Zhejiang, People’s Republic of China). 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR) was obtained from Molecular Probes Inc., (Eugene, OR, USA). Fetal bovine serum (FBS) was purchased from Sijiqing Biologic Co., Ltd., (Zhejiang, People’s Republic of China). All other chemicals were of analytical grade and used without further purification.
Synthesis of chitosan-g-stearate (CS)
As reported previously, the CS copolymer was synthesized via the reaction of the carboxyl groups of stearate (SA) with the primary amino groups of chitosan, catalyzed by EDC.3 Briefly, chitosan (17.5 KDa; 1.0 g) was dissolved in 60 mL super-purified water, and SA with EDC was dissolved in 40 mL ethanol by sonication treatment, followed by stirring in a water bath at 60°C for 30 minutes. The activated SA was added into the chitosan solution with vigorous stirring and the reaction was conducted for another 24 hours at 80°C. The final reactant was dialyzed using dialysis membrane (molecular weight cutoff 7 KDa; Spectrum Laboratories, Laguna Hills, CA, USA) against super-purified water for 2 days to remove the water-soluble byproducts, followed by freeze drying. The lyophilized product was then washed thrice with ethanol to remove unreacted SA. Finally, the CS product was dispersed in deionized water and lyophilized.
Synthesis of TNYL-conjugated CS (T-CS)
CS was further conjugated with TNYL peptide. Firstly, (Boc)2O [TNYL:(Boc)2O=1:5.2, mol/mol] was added into anhydrous dimethylformamide containing 10 mg TNYL in an ice bath, followed by stirring with light protection at room temperature for 12 hours. Then, NH2-PEG2000-NH2 (11.9 mg) and EDC (11.4 mg) were added into the above solution for another 24 hours to obtain t-Boc-TNYL-PEG2000-NH2. Pyridine (220 μL) and CA (1.86 mg) in 238.5 μL of dioxane were then added dropwise to the pre-cooling solution followed by another 6 hours at 4°C. Chilled diethyl ether was added and the precipitate was isolated by centrifugation and vacuum drying overnight to produce the intermediate t-Boc-TNYL-PEG2000-CA-COOH. Finally, t-Boc-TNYL-PEG2000-CA-COOH, EDC (11.4 mg), and N-hydroxysuccinimide (6.9 mg) were dissolved in deionized water and stirred for 1 hour to activate the carboxylic acid. CS (100 mg) was added and the pH value of the reaction mixture was adjusted to 7.0. After reaction for 24 hours, the protection of (Boc)2O to TNYL peptide was removed by adding hydrochloric acid. The resulting product (T-CS) was purified by dialysis (molecular weight cut-off: 7 kDa) against deionized water for 2 days, followed by lyophilization.
Preparation of DOX-loaded CS, T-CS micelles
DOX base was obtained by reaction of doxorubicin hydrochloride (DOX.HCl) with double mole triethylamine in dimethyl sulfoxide (DMSO) overnight.19 The DOX/DMSO stock solution (DOX concentration was 1 mg/mL) was added dropwise into the CS and T-CS micelle solutions (DOX: polymers =5%, weight per weight). The entire mixture was subjected to dialysis using a membrane (molecular weight cut-off 3.5 kDa, Spectrum Laboratories) against distilled water for 24 hours. The dialyzed products were centrifuged at 3,000 rpm for 8 minutes to remove the precipitated drug; the DOX-loaded micelle solutions were obtained, which were termed CS/DOX and T-CS/DOX.
Physicochemical characteristics of micelles
The H nuclear magnetic resonance (1H NMR spectra of the chemicals were obtained using an NMR spectrometer (AC-80; Bruker Biospin, Billerica, MA, USA). SA was dissolved in DMSO-D6; TNYL peptide, CS, and T-CS were dissolved in D2O at concentrations of 20 mg/mL.
The degrees of amino-substitution (SD%) for CS were determined by the 2,4,6-trinitrobenzene sulfonic acid (TNBS) method. Briefly, 2 mL of 100 mg/mL CS solution was incubated with 2 mL of 4% NaHCO3 and 2 mL of 0.1% TNBS solution for 2 hours at 37°C, followed by the addition of 2 mL of 2 M HCl. The ultraviolet (UV) absorbance of the mixture solution at 344 nm was measured by UV spectrophotometer (TU-180 0PC; Beijing Purkinje General Instrument Co., Ltd., Beijing, People’s Republic of China).
The critical micelle concentration (CMC) of CS and T-CS in deionized water was determined by the pyrene fluorescence method using a fluorometer (F-2500; Hitachi Ltd., Tokyo, Japan). A series of sample solutions with CS and T-CS concentrations ranging from 1.0 mg/mL to 10−4 mg/mL were sonicated at room temperature for 30 minutes. The solutions contained pyrene (6.0×10−7 mol/L). The slit openings were set at 10 nm (excitation) and 2.5 nm (emission), and the excitation wavelength was 337 nm. The CMC of CS was calculated by intensity ratio of the first highest energy bands to the third in the pyrene emission spectra.
The particle sizes of the CS, T-CS, CS/DOX, and T-CS/DOX micelles were measured by dynamic light scattering (Zetasizer 3000HS; Malvern Instruments Ltd., Malvern, UK). Distilled water was used for preparation of the micelles, with a final concentration of 1 mg/mL. Each sample determination was done in triplicate. The morphological examinations of the DOX-loaded micelles were performed by transmission electron microscopy (TEM; STEREOSCAN; Leica Microsystems, Wetzlar, Germany). The samples were dropped on copper grids with films for viewing with 2% (weight per volume) phosphotungstic acid staining.
Determination of drug encapsulation efficiency and drug loading
The doxorubicin content in the CS/DOX and T-CS/DOX micelles was measured using a fluorescence spectrophotometer. The excitation wavelength was set at 505 nm, emission wavelength at 565 nm, and slit openings at 5 nm. Briefly, CS/DOX and T-CS/DOX micelle solutions were diluted 100-fold by DMSO aqueous solution (DMSO:H2O =9:1, volume to volume), followed by ultrasound for 30 minutes to dissociate the micelles. Then, the DOX content was estimated by comparing to a standard curve. The drug encapsulation efficiency (EE%) and drug-loading content (DL%) can be calculated by Equations 1 and 2:
EE% = Me/Ma ×100%, | (1) |
DL% = Me/(Me + Mc) ×100%, | (2) |
where Me is the amount of DOX encapsulated in micelles, Ma is the amount of DOX added, and Mc is the amount of polymer micelles used.
Cell lines, cell culture, and MTT assay
Human ovarian carcinoma SKOV3 and human lung adenocarcinoma A549 cell lines were obtained from the Institute of Biochemistry and Cell Biology (Shanghai, People’s Republic of China). The cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% (volume per volume) FBS and penicillin/streptomycin (100 U/mL, 100 U/mL) at 37°C in a humidified atmosphere containing 5% CO2.
The MTT assay was used to evaluate the cytotoxicity of blank micelles and in vitro antitumor activity of DOX-loaded micelles in SKOV3 cell lines. Cells were seeded into 96-well plates at an initial density of 1×104 cells/well in 200 μL of RPMI 1640 complete medium. After 24 hours’ growth, the cells were exposed to fresh culture medium containing a series of concentrations of CS and DOX-loaded CS, or T-CS and DOX-loaded T-CS micelles. After 48 hours under standard incubation conditions, 20 μL of MTT reagent (an MTT stock solution of 5 mg/mL) solution was added to each well and incubated for an additional 4 hours. The medium was then removed and 200 μL of DMSO was added to each well to dissolve the formed purple formazan crystals. The plates were mildly shaken for 10 minutes to ensure the dissolution of formazan. The absorbance value was measured using a microplate reader (Model 680; Bio-Rad Laboratories Inc., Hercules, CA, USA). Three replicates were counted for each sample. The mean value was used as the final result.
Cellular uptake of micelles
FITC-labeled CS and T-CS micelles were synthesized according to the methods described in the literature.20 To remove the unconjugated FITC, the synthesized FITC-labeled micelles were dialyzed in the dark against deionized water followed by freeze drying. SKOV3 cells were seeded at a density of 105 cells/well in a 24-well plate (Nalge Nunc International, Penfield, NY, USA), and allowed to grow for 24 hours. The medium was then replaced with 1 mL of fresh culture medium containing FITC-labeled CS and T-CS micelles (100 μg/mL) and the cells were further incubated for 1, 6, and 24 hours. After rinsing the cells with phosphate-buffered saline (PBS) three times, cellular uptake was observed by an inverted fluorescence microscope (DMI 4000B; Leica). For the quantitative analysis of cell uptake, cells were treated with trypsin after 2 hours’ incubation with FITC-labeled CS and T-CS micelles, and then resuspended in PBS. The intensity of cellular fluorescence was determined by a flow cytometer (FC500MCL; Beckman Coulter, Brea, CA, USA).
The cellular uptake of the CS/DOX and T-CS/DOX micelles was studied using SKOV3 cells. Briefly, SKOV3 cells were seeded at a density of 105 cells/well in a 24-well plate and allowed to grow for 24 hours. The cells were then incubated with the CS/DOX and T-CS/DOX micelles for 2, 8, and 24 hours. After washing the cells with PBS three times, the cellular uptake was observed using an inverted fluorescence microscope (DMI 4000B; Leica).
In vitro cellular competitive uptake of DOX-loaded micelles
The in vitro targeting ability of the T-CS/DOX micelles was evaluated by competitive uptake in the co-culture of SKOV3 and A549 cells.21 Before incubation with DOX-loaded micelles, A549 cells were stained with PKH67 Fluorescent Cell Linker (Sigma-Aldrich Co.) following the supplier’s protocol.22 A549 cells were disaggregated and resuspended in 200 μL Diluent C, and then were incubated with 200 μL PKH67 dye (4 mM) for 10 minutes at room temperature. To stop the staining reaction, the cells were incubated with 1 mL of serum for 2 minutes. The stained cell pellet was obtained by centrifugation at 400 g for 10 minutes, followed by washing two more times with 10 mL of complete medium to ensure removal of unbound dye. Finally, the cells were resuspended to the desired concentration.
Both SKOV3- and PKH67-labeled A549 cells were cultured in the same wells in a 24-well plastic plate with the same concentration of cells and incubated for 24 hours to attach. Then, CS/DOX and T-CS/DOX micelles was added into the wells at a concentration of 20 μg/mL. For the blocking experiments, cells were firstly incubated with free TNYL (1.0 mmol/L) for 0.5 h and then with T-CS/DOX micelles. After incubation for 1 hour, the cells were washed with PBS three times, and the cellular uptake was observed by confocal laser scanning microscopy (IX81-FV1000; Olympus Corporation, Tokyo, Japan).
In vivo distribution studies in tumor-bearing mice
All animal procedures were performed according to national regulations and approved by the local animal experiments ethical committee.
The DiR was used as a fluorescent probe23 and was loaded into the T-CS micelles for in vivo fluorescent imaging investigation. The xenografted tumor models were established by subcutaneous injection of SKOV3 cells (5×106 cells in 100 μL of serum-free Dulbecco’s Modified Eagle’s Medium) into the left flank and A549 cells (5×106 cells in 100 μL of serum-free Dulbecco’s Modified Eagle’s Medium) into the right flank of male BALB/C+nu/F1 nude mice. After the tail vein injection of T-CS/DiR micelles which contained 0.5 mg polymers and 4.9 μg, DiR the mice were anesthetized by diethyl ether and were observed by the Maestro in vivo imaging system (CRI Inc., Woburn, MA, USA) at the predetermined time. For ex vivo analysis, the mice were sacrificed and excised tissues together with the tumors were collected and weighed. The fluorescent intensity, responding to the amount of micelles, was also read by the imaging system. The accumulation of T-CS/DiR in various tissues was expressed as the percentage of the injected dose per gram of tissue (% ID/g).
Statistical analysis
Results were reported as means ± standard deviation. Paired t-tests were applied to assess the statistical difference among the different groups. Differences were considered statistically significant when P<0.05.
Results
Synthesis and characteristics of T-CS
CS was synthesized by the coupling reaction of SA and chitosan in the presence of EDC according to previous reports.24 The actual substitution degree (SD) of stearic acid was 5.4%, as measured by the TNBS assay. Figure 1A presents the synthesis scheme of T-CS. After pre-protection the amino terminus of TNYL with (Boc)2O, the coupling reaction between the carboxyl group of TNYL and the amino groups of PEG was catalyzed by EDC. Then, the t-Boc-TNYL-PEG-CA-COOH was obtained by conjugating CA to the end amino group of PEG according to our previous study,6,25 with some modification. The final product, T-CS, was synthesized by coupling the carboxyl group of t-Boc-TNYL-PEG-CA-COOH and the remaining primary amino groups in CS in the presence of EDC and N-hydroxysuccinimide.
The chemical structures of the obtained CS and T-CS were confirmed by 1H NMR spectra (Figure 1B). The peaks at about 0.95–1.08 ppm were attributed to the methylene and methyl hydrogen for SA and chitosan, respectively. Compared with CS, the 1H NMR spectrum of T-CS showed a sharp single peak at about 1.16–1.26 ppm, which was attributed to the methylene hydrogen proton peak of the proline in TNYL. These results indicate that T-CS was synthesized successfully.
After dispersing the CS and T-CS in distilled water, the micelles were easily prepared due to their inherent self-aggregation in aqueous environments. The aggregation behaviors of CS and T-CS in aqueous media were investigated by fluorescence spectroscopy with pyrene as a fluorescent probe.26 As shown in Figure 2A, the CMC values of CS and T-CS were determined to be 81.0 μg/L and 91.2 μg/L, respectively. The sizes of the CS and T-CS micelles formed in aqueous media at the concentration of 1.0 mg/mL were determined to be 31.6±0.9 nm and 82.1±2.8 nm, respectively. The slightly increased size of T-CS may be attributed to the introduction of PEG and the TNYL hydrophilic chain, which stretches toward the water phase. Figures 2B and C show the size distribution of CS and T-CS micelles at 1.0 mg/mL concentration. The zeta potentials of the CS and T-CS micelles were determined to be 36.2±1.1 mV and 30.9±0.5 mV. The reduced amino groups after TNYL grafting may have led to the slightly decreased zeta potential.
Preparation and characteristics of DOX-loaded micelles
The CS/DOX and T-CS/DOX micelles were prepared successfully by the dialysis method. The mean micelle size, polydispersity index of size, drug entrapment efficiency, and drug-loading content of the CS/DOX and T-CS/DOX micelles are shown in Table 1. The micelle sizes of T-CS/DOX were smaller than 100 nm. There was no obvious difference in the particle sizes observed after the drug loading. It was reported that the total cellular uptake of nanoparticles sub-100 nm (40.6 nm) was almost 12-fold that of the largest ones (162.1 nm and 276.6 nm).27 Particles of about 100 nm are known to be suitable for intravenous application and can achieve long plasma circulation times.28 The DOX-loaded micelles were spherical in shape as observed by TEM (Figure 3) and the average sizes were accordance with those obtained by dynamic light scattering. When the drug-feeding amount was fixed at 5.00% (weight per weight), the DL% of DOX-loaded micelles ranged from 3.43%–4.35% (Table 1). The drug EE% of T-CS/DOX reached up to 86.43%.
  | Figure 3 TEM images of CS/DOX and T-CS/DOX micelles. |
Cellular internalization ability of micelles
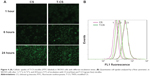
The cellular uptake test was then carried out using SKOV3 cells with high EphB4 receptor expression.16,29 Figure 4A shows fluorescent microscopic images after FITC-labeled blank micelles were incubated with SKOV3 cells for 1, 6, and 24 hours. The cellular uptake of the CS and T-CS micelles increased with prolonged incubation time. The obvious fluorescence could be detected in all cells at 24 hours. However, a noteworthy feature was that fluorescence intensity of the T-CS micelles in SKOV3 cells was stronger than that of the CS micelles with a short incubation time (1 hour). Furthermore, the intracellular uptake of the FITC-labeled micelles was also conducted in a quantitative way. The average fluorescent intensity of the T-CS micelles as measured by flow cytometry after 1 hour of incubation was 9.98, which was higher than that of the CS micelles (8.21; Figure 4B). This difference reduced with increasing incubation time.
The CS and T-CS micelles could get into tumor cells with extension of the incubation time.3 Also, the T-CS micelles with TNYL conjugation could target the EphB4 receptor on the cell surface. Therefore, the fluorescence intensity of the T-CS micelles in the SKOV3 cells was stronger than that of the CS micelles.
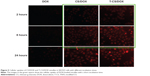
The cellular uptake of the CS/DOX and T-CS/DOX micelles in SKOV3 cells was also tested. As shown in Figure 5, the fluorescence of the T-CS/DOX micelles was stronger than that of the CS/DOX micelles at 2 and 8 hours of incubation. It was revealed that DOX can be transferred into tumor cells by the micelles. Also, more DOX accumulation can be obtained after TNYL conjugation.
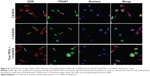
Western blotting analysis and immunostaining assay were used by You et al to confirm the high expression levels of EphB4 receptor in the SKOV3 cells, but low expression levels in the A549 cells.29 To intensively investigate the tumor-specific delivery of the micelles in vitro, cellular competitive uptake of DOX-loaded micelles on EphB4-positive SKOV3 cells and EphB4-negative A549 cells’ co-cultured systems was tested within 1 hour and observed using confocal laser scanning microscopy. A549 cells, stained with the green fluorescence probe PKH67, were distinguished from SKOV3 cells. As shown in Figure 6, the antitumor drug DOX can be internalized into cancer cells via mediation by the micelles. The CS/DOX micelles without TNYL conjugation indicate similar uptake on the cells’ co-cultured systems. The fluorescent intensity in the SKOV3 cells (Figure 6, white arrows) was obviously higher than that in the A549 cells (Figure 6, yellow arrows) for T-CS/DOX micelles, which may be due to specific binding of TNYL to the EphB4 receptor on the cell surface. Furthermore, after the EphB4 receptors were blocked with the addition of free TNYL peptide, the cell uptake of the T-CS/DOX micelles showed no visible difference between the two cell lines. This demonstrated that the uptake selectivity of the T-CS micelles between those two cell lines was obtained by TNYL peptide–mediated endocytosis after the targeting modification.
Nanoparticles mainly enter into cells by endocytosis. The smaller the size of the nanoparticles, the faster the cellular internalization.27 The size of T-CS/DOX was larger than that of CS/DOX (Table 1). However, the cellular uptake of T-CS/DOX was faster (Figure 4). Also, after the EphB4 receptor was blocked by adding free TNYL, T-CS/DOX did not show improved cell targeting selectivity (Figure 6) between SKOV3 and A549 cells in the cellular competitive uptake study. Thus, it was the TNYL conjugation that led to the increased cellular uptake.
In vitro cytotoxicity assay
In vitro cytotoxicity and antitumor activity were measured by the MTT assay. Figure 7A shows the cellular growth inhibition of SKOV3 cells after incubation with blank CS and T-CS micelles for 48 hours. The half maximal inhibitory concentration (IC50) values of CS and T-CS showed no significant differences, and were 445±16.5 μg/mL and 412±13.0 μg/mL, respectively. It was shown that the blank micelles had low cytotoxicity. After loading DOX, the cytotoxicity of the T-CS/DOX micelles against the SKOV3 cells obviously increased, especially when the concentration of DOX was low, as shown in Figure 7B. The IC50 value was about 0.8 μg/mL, which increased the cytotoxicity by approximately 2.3-fold compared with CS/DOX (1.85 μg/mL). The mechanisms of the improved cytotoxicity for DOX loaded in micelles has been researched and discussed previously.30 When the concentration of DOX was 2 μg/mL, the amounts of CS and T-CS blank micelles, calculated by DL% formulas, were only about 58 μg/mL and 46 μg/mL, respectively. Thus, it is clear that the enhanced cytotoxicity is due to the increased intracellular drug concentration. The uptake can reach saturation with prolonged study time. Prior to cellular uptake reached saturation, the faster the cellular uptake, the longer interaction between anticarcinogen and tumor cells. Owing to the special function and surface cationic charge property of micelles, DOX can get into cancer cells increasedly when entrapped by micelles. In addition, the enhanced uptake of T-CS/DOX is mediated by the specific interaction between TNYL and EphB4 receptor. It may be that TNYL can combine EphB4 receptors on the SKOV3 cells’ surface and can facilitate the internalization of the micelles by receptor-mediated endocytosis.5 The above results further show the potent ovarian carcinoma-targeting ability of TNYL-modified CS.
In vivo imaging of micelles in tumor-bearing mice
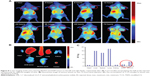
In order to investigate the targeting behavior of the T-CS micelles further, we used in vivo experiments with tumor-bearing mice. The tumor model was built by simultaneous subcutaneous injection of SKOV3 and A549 cells in the left and right flanks of the mice, respectively. To track the location of the micelles, near-infrared–emitting DiR was loaded into the T-CS micelles. Biodistribution and tumor selective accumulation were then analyzed by in vivo imaging after intravenous injection of the T-CS/DiR micelles. It was obvious that fluorescence accumulation of the T-CS/DiR micelles in the SKOV3 tumors was more significant than in the A549 tumors during the whole experimental process, which maximized 72 hours post-injection (Figure 8A). The fluorescence intensity of various tissues 72 hours after injection was observed and quantified (Figures 8B and C). These results further confirmed the 2.5-fold difference of fluorescence intensity between the SKOV3 and A549 tumors. The in vivo distribution of CS/DiR was also carried out as a control (data not shown). There was no significant difference between the fluorescence accumulations in the two kinds of tumors. This result shows that the T-CS micelles conjugated to TNYL had better selectivity to SKOV3 tumors with positive-EphB4 expression than A549 tumors with negative-EphB4 expression.
The chemical information communication of cells depends on the ability of signal molecules, secretion, and mutual recognition of receptors and ligands. Receptor – ligand binding is a highly specific reaction. The targeting modification of T-CS with TNYL can achieve significant tumor selectivity. This result was consistent with the in vitro cellular uptake (Figure 4B), which demonstrated that the uptake efficiency changed slightly from red to green lines with the targeting modification. T-CS had higher ovarian tumor targeting in virtue of the ligand modification. Furthermore, compared with some synthetic polymers, T-CS is considered to be less toxic and biodegradable; therefore, it has good biocompatibility and low long-term toxicity due to the peptide-like linkage between the monomers.
Conclusion
The CS-based polymer micelles with TNYL-targeting modification were successfully synthesized and developed as an efficient vector for antitumor drug delivery. T-CS can form micelles by self-aggregation in the aqueous medium with a low CMC value. The micelles showed excellent DOX-packaging capability. The cellular uptake and antitumor activity of the T-CS–based drug delivery system was significantly enhanced in EphB4-positive cells due to increased endocytosis mediated by the targeting molecule TNYL. Both in vitro and in vivo tests showed the tumor-specific delivery due to the active-targeting modification. These results demonstrate that the T-CS micelles are a promising candidate for active-targeting drug delivery.
Acknowledgments
We appreciate the financial support of the National Nature Science Foundation of China (81200428) and the Nature Science Foundation of Zhejiang province (LY12H30007 and Y2090336).
Disclosure
The authors report no conflicts of interest in this work.
References
Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010; 6(6):714–729. | ||
Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev. 2010;62(1):28–41. | ||
Hu FQ, Zhao MD, Yuan H, You J, Du YZ, Zeng S. A novel chitosan oligosaccharide-stearic acid micelles for gene delivery: properties and in vitro transfection studies. Int J Pharm. 2006;315(1–2):158–166. | ||
Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;162(1–2):1–16. | ||
Liu Y, Li J, Shao K, et al. A leptin derived 30-amino-acid peptide modified pegylated poly-L-lysine dendrigraft for brain targeted gene delivery. Biomaterials. 2010;31(19):5246–5257. | ||
Hu FQ, Liu LN, Du YZ, Yuan H. Synthesis and antitumor activity of doxorubicin conjugated stearic acid-g-chitosan oligosaccharide polymeric micelles. Biomaterials. 2009;30(36):6955–6963. | ||
Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–131. | ||
Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed Engl. 2003;42(38):4640–4643. | ||
Wei Z, Hao J, Yuan S, et al. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009;376(1–2):176–185. | ||
Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. | ||
Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–146. | ||
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. | ||
Muro S. Challenges in design and characterization of ligand-targeted drug delivery systems. J Control Release. 2012;164(2):125–137. | ||
Erber R, Eichelsbacher U, Powajbo V, et al. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. Embo J. 2006;25(3):628–641. | ||
You J, Zhang R, Xiong C, et al. Effective photothermal chemotherapy using doxorubicin-loaded gold nanospheres that target EphB4 receptors in tumors. Cancer Res. 2012;72(18):4777–4786. | ||
Ma X, Luo D, Li K, Liu R, Liu Y, Zhu T, et al. Suppression of EphB4 improves the inhibitory effect of mTOR shRNA on the biological behaviors of ovarian cancer cells by down-regulating Akt phosphorylation. J Huazhong Univ Sci Technolog Med Sci. 2012;32(3):358–363. | ||
Koolpe M, Burgess R, Dail M, Pasquale EB. EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J Biol Chem. 2005;280(17):17301–17311. | ||
Ye YQ, Chen FY, Wu QA, et al. Enhanced cytotoxicity of core modified chitosan based polymeric micelles for doxorubicin delivery. J Pharm Sci. 2009;98(2):704–712. | ||
Kohori F, Yokoyama M, Sakai K, Okano T. Process design for efficient and controlled drug incorporation into polymeric micelle carrier systems. J Control Release. 2002;78(1–3):155–163. | ||
Du YZ, Cai LL, Li J, et al. Receptor-mediated gene delivery by folic acid-modified stearic acid-grafted chitosan micelles. Int J Nanomedicine. 2011;6:1559–1568. | ||
Du YZ, Cai LL, Liu P, You J, Yuan H, Hu FQ. Tumor cells-specific targeting delivery achieved by A54 peptide functionalized polymeric micelles. Biomaterials. 2012;33(34):8858–8867. | ||
Martina MS, Wilhelm C, Lesieur S. The effect of magnetic targeting on the uptake of magnetic-fluid-loaded liposomes by human prostatic adenocarcinoma cells. Biomaterials. 2008;29(30):4137–4145. | ||
Texier I, Goutayer M, Da Silva A, et al. Cyanine-loaded lipid nanoparticles for improved in vivo fluorescence imaging. J Biomed Opt. 2009; 14(5):054005. | ||
Hu FQ, Li YH, Yuan H, Zeng S. Novel self-aggregates of chitosan oligosaccharide grafted stearic acid: preparation, characterization and protein association. Pharmazie. 2006;61(3):194–198. | ||
Hu FQ, Zhang YY, You J, Yuan H, Du YZ. pH triggered doxorubicin delivery of PEGylated glycolipid conjugate micelles for tumor targeting therapy. Mol Pharm. 2012;9(9):2469–2478. | ||
Kalyanasundaram K, Thomas JK. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc. 1977;99(7):2039–2044. | ||
Andar AU, Hood RR, Vreeland WN, Devoe DL, Swaan PW. Microfluidic preparation of liposomes to determine particle size influence on cellular uptake mechanisms. Pharm Res. 2014;31(2):401–413. | ||
Ocal H, Arica-Yegin B, Vural I, Goracinova K, Caliş S. 5-Fluorouracil-loaded PLA/PLGA PEG-PPG-PEG polymeric nanoparticles: formulation, in vitro characterization and cell culture studies. Drug Dev Ind Pharm. 2014;40(4):560–567. | ||
You J, Wang Z, Du Y, et al. Specific tumor delivery of paclitaxel using glycolipid-like polymer micelles containing gold nanospheres. Biomaterials. 2013;34(18):4510–4519. | ||
Zhao MD, Hu FQ, Du YZ, et al. Coadministration of glycolipid-like micelles loading cytotoxic drug with different action site for efficient cancer chemotherapy. Nanotechnology. 2009;20(5):055102. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.