Back to Journals » OncoTargets and Therapy » Volume 8
The role of 18F-FDG PET/CT imaging in patient with malignant PEComa treated with mTOR inhibitor
Authors Sun L, Sun X, Li Y, Xing L
Received 25 March 2015
Accepted for publication 18 June 2015
Published 30 July 2015 Volume 2015:8 Pages 1967—1970
DOI https://doi.org/10.2147/OTT.S85444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Faris Farassati
Lu Sun,1 Xiaorong Sun,2 Yuhui Li,3 Ligang Xing4
1School of Medicine and Life Sciences, University of Jinan-Shandong Academy of Medical Sciences, 2PET/CT Center, Department of Radiology, 3Department of Pathology, 4Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Jinan, Shandong, People’s Republic of China
Abstract: Malignant perivascular epithelioid cell tumor (malignant PEComa) is a rare disease for which the diagnostic criteria and treatment options have not been established. Since PEComa is associated with upregulation of mammalian target of rapamycin (mTOR) pathway which controls Glut-1 (glucose transporter) function, increased 18F-fluorodeoxyglucose (18F-FDG) uptake may indicate the over activation of mTOR pathway and may guide selectively inhibiting mTOR pathway treatment. We report a malignant PEComa patient who presented for 18F-FDG positron emission tomography/computed tomography (PET/CT) restaging. The tumor had shown significant avidity on PET/CT as well as an evident response to sirolimus (rapamycin, Rapamune™) that supports the utility of mTOR inhibitors as an effective treatment for malignant PEComa. Therefore, 18F-FDG PET/CT is helpful in restaging and guiding treatment for malignant PEComa with mTOR inhibitors.
Keywords: malignant perivascular epithelioid cell tumor, PEComa, mTOR inhibitor, FDG, PET/CT
Introduction
Perivascular epithelioid cell tumor (PEComa) is a mesenchymal tumor composed of distinctive cells that show a focal association with blood vessel walls and usually express melanocytic and smooth muscle markers.1 PEComa arising at soft-tissue and visceral sites is rare, with approximately 200 reported cases worldwide. Approximately one-third of the PEComas show locally aggressive behavior, and are called “malignant PEComa”.2 Since few cases have been reported, there is still no consensus about imaging/pathology diagnostic characteristics, staging/restaging, and treatment options for the management of malignant PEComa.1,3 PEComa is driven by a tuberous sclerosis complex gene mutation causing upregulation of mammalian target of rapamycin (mTOR) pathway. Selectively inhibiting mTOR pathways has shown efficiency in malignant PEComa.4 Meanwhile, mTOR pathway controls multiple cellular processes, especially Glut-1 (glucose transporter) function.5 Therefore, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) may have potential in diagnosing, staging/restaging, and guiding treatment of malignant PEComa with mTOR inhibitors by detecting the increased glucose metabolism in tumor. To our knowledge, no report has been published about the role of 18F-FDG PET/CT in patients with malignant PEComa treated with mTOR inhibitors. In this report, we present a case of malignant uterus PEComa with intense FDG uptake in disseminating metastatic foci, successfully treated with sirolimus (rapamycin, Rapamune™), an mTOR inhibitor.
Case report
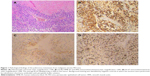
A 46-year-old female presented to our institution with solitary renal and pulmonary lesions 2 years after the initial diagnosis of uterine leiomyosarcoma. Left nephrectomy and right lower lung lobe wedge resection were performed. Histological and immunohistochemical analysis of the renal and pulmonary lesions, in addition to retrospective re-evaluation of the initial uterine tumor, led to the final diagnosis of malignant uterine PEComa with late renal and pulmonary metastases. Pulmonary metastases from malignant uterine PEComa display nests of epithelioid cells with small round nuclei surrounding thin-walled capillary vessels (Figure 1A). Tumor shows diffusely positive staining for melanocytic marker and smooth muscle marker (Figure 1B and C). A high Ki-67 labeling index of 40% indicates the aggressive behavior of the tumor (Figure 1D).
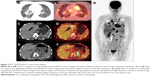
18F-FDG PET/CT scan was performed 1 month after pulmonary and kidney surgery to restage the disease. The PET/CT imaging showed bilateral multiple pulmonary metastases with maximum standardized uptake value (SUVmax) of 6.5 (circles in Figure 2A and B), liver metastases with SUVmax of 12.1 (arrows in Figure 2C and D), metastatic foci growing from previous left kidney bed with SUVmax of 7.3 (boxes in Figure 2E and F). In addition, whole body FDG PET showed multiple distant metastases, including the disseminating tumor emboli with intense FDG uptake (Figure 2G).
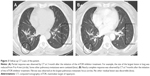
The patient was treated with sirolimus (rapamycin, Rapamune™; Wyeth, Madison, NJ, USA), an inhibitor of mTOR. Serial CT scans were performed to monitor the treatment response. The follow-up axial CT images (Figure 3A and B) showed that the pulmonary metastatic foci were gradually diminishing and eliminating. A favorable and sustained response was observed at 7 months after the PET/CT scanning. The patient continued using the mTOR inhibitors untill the last follow-up. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Discussion
The PEComa family includes lymphangioleiomyomatosis, angiomyolipoma, clear cell sugar tumor of the lung; and a group of histologically and immunophenotypically similar tumors arising at a variety of soft-tissue and visceral sites. PEComas other than lymphangioleiomyomatosis and angiomyolipoma are rare, especially “malignant PEComa” which is accounting for one-third of the PEComa. The consensus about the diagnostic criteria and treatment options for malignant PEComa have not been established.1
18F-FDG PET/CT has shown the potential role in differentiating malignant and benign PEComas.2,6–13 Previous reports have shown that all the malignant PEComas (n=4) presented with intense FDG uptake at both primary lesions and metastatic foci with SUVmax ranging from 3.19 to 72.2,6–8 However, 55 out of 62 benign PEComas have exhibited low or negative FDG uptake on PET imaging with SUVmax lower than 2.0.9–13 Since PEComa is driven by tuberous sclerosis complex gene mutation causing upregulation of mTOR pathway which controls multiple cellular processes, including Glut-1 function,5 high FDG uptake in malignant PEComa could reflect over activation of mTOR pathway and is useful for staging/restaging in clinic.
Pretreatment FDG PET/CT has shown the ability in predicting outcome of treatment with chemotherapy, radiotherapy, and targeted therapy in variable cancer. In general, the patients with higher FDG uptake were associated with poorer prognosis compared to those with lower FDG uptake. Similarly, malignant PEComa patients with intense FDG uptake in previous case reports all failed to chemo- or radiotherapy.6,7 For example, Ciarallo et al reported a malignant PEComa revealed by FDG PET/CT that were resistant to radiotherapy, chemotherapy, and imatinib mesylate.7 At the same time, prior studies demonstrated that mTOR inhibitors such as sirolimus may be recommended in this rare disease type.4,5 Based on these evidences, the patient was treated with mTOR inhibitor and got a nearly complete clinical response. Hence, we presumed that if increased glucose metabolism in malignant PEComa was linked with the upregulation of mTOR pathway, high FDG uptake may suggest that targeting on inhibiting mTOR pathway would be an effective treatment as shown in our case. However, one case from Dickson et al’s study, the only case as far as we knew, showed resistance to sirolimus although with avid FDG uptake.14 Indeed, it still needs more patients to establish a general statement. Further studies are required with functional imaging including FDG PET/CT to understand the determinants of response and mechanisms of acquired resistance.
Conclusion
18F-FDG PET/CT is helpful in staging/restaging for malignant PEComa and guiding treatment with mTOR inhibitors.
Acknowledgment
This work is supported by grants (NSFC 81272502, ZR2014YL033, and 2014WS0030).
Disclosure
The authors report no conflicts of interest in this work.
References
Hornick JL, Pan CC. PEComa. In: Fletcher CD, Bridge J, Hogendoorn P, Mertens F, editors. World Health Organization Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon: International Agency for Research on Cancer; 2013:230–231. | ||
Armah HB, Parwani AV. Malignant perivascular epithelioid cell tumor (PEComa) of the uterus with late renal and pulmonary metastases: a case report with review of the literature. Diagn Pathol. 2007;2:45. | ||
Tirumani SH, Shinagare AB, Hargreaves J, et al. Imaging features of primary and metastatic malignant perivascular epithelioid cell tumors. AJR Am J Roentgenol. 2014;202(2):252–258. | ||
Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28(5):835–840. | ||
Benson C, Vitfell-Rasmussen J, Maruzzo M, et al. A retrospective study of patients with malignant PEComa receiving treatment with sirolimus or temsirolimus: the Royal Marsden Hospital experience. Anticancer Res. 2014;34(7):3663–3668. | ||
Navarro-Pelayo Lainez MM, Ramos-Font C, Rebollo Aguirre AC, Rodriguez-Fernandez A, Llamas-Elvira JM. Perivascular epithelioid tumors: Utility of the positron emission tomography with 18F-fluorodesoxyglucose (PET-TAC FDG) in their staging and follow-up. Rev Esp Med Nucl. 2010;29(5):258–262. | ||
Ciarallo A, Makis W, Hickeson M, Derbekyan V. Malignant perivascular epithelioid cell tumor (PEComa) of the uterus: serial imaging with F-18 FDG PET/CT for surveillance of recurrence and evaluation of response to therapy. Clin Nucl Med. 2011;36(4):e16–e19. | ||
Rakheja R, Abikhzer G, Alabed YZ, Nahal A, Lisbona R. The appearance of osseous PEComa on F-18 FDG PET/CT. Clin Nucl Med. 2012;37(2):190–192. | ||
Welling RD, Lungren MP, Coleman RE. Extrarenal retroperitoneal angiomyolipoma mimicking metastatic melanoma: CT and FDG PET correlation. Clin Nucl Med. 2012;37(7):705–706. | ||
Ho CL, Chen S, Ho KM, et al. Dual-tracer PET/CT in renal angiomyolipoma and subtypes of renal cell carcinoma. Clin Nucl Med. 2012;37(11):1075–1082. | ||
Young LR, Franz DN, Nagarkatte P, et al. Utility of [18F]2-fluoro-2-deoxyglucose-PET in sporadic and tuberous sclerosis-associated lymphangioleiomyomatosis. Chest. 2009;136(3):926–933. | ||
Dong A, Wang Y, Zuo C. Synchronous pure epithelioid angiomyolipoma of the kidney and retroperitoneal schwannoma in the same patient on 18F-FDG PET/CT imaging. Clin Nucl Med. 2013;38(2):e98–e100. | ||
Kumasaka S, Arisaka Y, Tokue A, Higuchi T, Nakajima T, Tsushima Y. A case of multiple hepatic angiomyolipomas with high (18) F-fluorodeoxyglucose uptake. BMC Med Imaging. 2014;14:17. | ||
Dickson MA, Schwartz GK, Antonescu CR, Kwiatkowski DJ, Malinowska IA. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: clinical and molecular correlates. Int J Cancer. 2013;132(7):1711–1717. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.