Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 9 » Issue 1
The relationship between early reversibility test and maximal inspiratory pressure in patients with airway obstruction
Authors Ozkaya , Dirican A, Kaya S, Karanfil R, Bayrak M, Bostancı O, Ece F, Sengul B
Received 2 December 2013
Accepted for publication 10 March 2014
Published 5 May 2014 Volume 2014:9(1) Pages 453—456
DOI https://doi.org/10.2147/COPD.S58584
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Sevket Ozkaya,1 Adem Dirican,2 Sule Ozbay Kaya,3 Rabia C Karanfil,3 Merve G Bayrak,4 Ozgür Bostancı,5 Ferah Ece1
1Department of Pulmonary Medicine, Faculty of Medicine, Bahcesehir University, Istanbul, 2Department of Pulmonary Medicine, 3Department of Physical Therapy and Rehabilitation Clinic, Samsun Medicalpark Hospital, 4Department of Pulmonary Medicine, Samsun Chest Diseases and Thoracic Surgery Hospital, 5Academy of Sports, Ondokuzmayis University, Samsun, Turkey
Abstract: Maximal inspiratory pressure (MIP) is a marker for assessing the degree of respiratory muscle dysfunction. Muscle dysfunction represents a pathophysiological feature of chronic obstructive pulmonary disease. We aimed to determinate the MIP value in patients with airway obstruction, to evaluate the change in MIP with bronchodilator drug, and to show the relationship between the changes in MIP and disease characteristics. We evaluated 21 patients with airway obstruction at the Department of Pulmonary Medicine, Samsun Medicalpark Hospital, Samsun, Turkey. We performed pulmonary function tests, measurement of MIP values, and reversibility tests with salbutamol. The baseline spirometry results were: mean forced vital capacity (FVC), 3,017±1,020 mL and 75.8%±20.8%; mean forced expiratory volume in 1 second (FEV1), 1,892±701 mL and 59.2%±18.2%; FEV1/FVC, 62.9%±5.5%; peak expiratory flow, 53%±19%. The pre-bronchodilator MIP value was 62.1±36.9 cmH2O. The reversibility test was found to be positive in 61.9% of patients with salbutamol. The absolute change and percentage of change in FEV1 were 318±223 mL and 19.8%±16.7%, respectively. The MIP value was increased by 5.5 cmH2O (8.8%) and was 67.7±30.3 cmH2O after bronchodilation. There was no significant relationship between age, FEV1, reversibility, and change in MIP with bronchodilator. However, the increase in MIP with bronchodilator drug was higher in patients with low body mass index (<25 kg/m2). We noted a 13.1% increase in FVC, a 19.8% increase in FEV1, a 20.2% increase in peak expiratory flow, and an 8.8% increase in MIP with salbutamol. In conclusion; MIP increases with bronchodilator therapy, regardless of changes in lung function, in patients with airway obstruction. The reversibilty test can be used to evaluate change in MIP with salbutamol.
Keywords: asthma, COPD, maximal inspiratory pressure, MIP, reversibility test, salbutamol
Introduction
Lung hyperinflation is a consequence of airway obstruction, increased airway resistance, and treatment to compliance in patients with chronic obstructive pulmonary disease (COPD), which may result in respiratory muscle weakness. Muscle dysfunction represents a pathophysiological feature of COPD. According to reported articles, Maximal inspiratory pressure (MIP) is a marker for assessing the degree of respiratory muscle dysfunction. The measurement of maximum static mouth pressures, made against an occluded airway – MIP and maximal expiratory pressure (MEP) – is the most widely used, and one simple way to gauge power of respiratory muscles and quantify the severity of disease. MIP and MEP values were lower in patients with severe obstruction, compared with healthy subjects. MIP decreased in patients with mild and moderate functional impairment.1–4
In patients found to have airway obstruction, evaluation of acute response to bronchodilators – the test of reversibility of airway obstruction – is a commonly-used procedure in clinical and research studies. Salbutamol is a short-acting β2-adrenergic receptor agonist (SABA) used for the treatment and assesement of early reversibilty in patients diagnosed as having obstructive lung disease. Usually, forced expiratory volume in 1 second (FEV1) or forced vital capacity (FVC) values, before and after administration of the bronchodilator, are compared and the change computed.5
We aimed to 1) show the determination of MIP values in patients with airway obstruction, 2) evaluate the change in MIP with bronchodilation, and 3) show the relationship between the changes in MIP and disease characteristics.
Materials and methods
We evaluated 21 patients at the Department of Pulmonary Medicine, Samsun Medicalpark Hospital, Samsun, Turkey, who met the inclusion criteria specified below:
- Currently symptomatic (cough, dyspnea, and/or wheezing);
- Presence of airway obstruction in spirometry (FEV1/ FVC ≤70% of expected value);
- Had never used bronchodilators before; and
- Had not received short- or long-acting inhaled bronchodilator therapy within the previous 12 hours.
We performed pulmonary function tests, measurement of MIP values, and reversibility tests with salbutamol.
Pulmonary function test and reversibility assessment
Pulmonary function tests were performed according to European Respiratory Society standards.6,7 Basal FEV1 and FEV1/FVC values were measured by the same physician using the MIR MiniSpir® PC-Based USB Spirometer (MIR Medical International Research, Waukesha, WI, USA) in an outpatient clinic setting following a 30-minute resting period. The test was performed in the seated position with the nose clamped and nasal respiration hindered. Patients performed the forced expiratory maneuver at least three times, and the maximum FEV1 value was recorded as the basal value.
Measurement of MIP
A MicroRPM respiratory pressure meter (Micro Medical, Chatham, UK) was used to measure respiratory muscle strength. MIP was measured from residual volume and the MEP was measured from total lung capacity. The patient should maintain inspiratory pressure for at least 1 (or up to 3) seconds, and the greatest negative pressure sustained for at least 1 second (not a transient spike) should be recorded. These durations are estimated by the individual supervising the test. The patient should rest for about 1 minute, and the maneuver should be repeated five times. Pre- and post-bronchodilator values were recorded.
Reversibility test
Following baseline spirometry, subjects inhaled salbutamol (Ventolin®; GlaxoSmithKline, London, UK) (100 μg =4 inhalations; total dose =400 μg) administered using a pressurized metered-dose inhaler with a spacer (Ventolin®; GlaxoSmithKline). Spirometry was performed 15 minutes later. Reversibility levels were evaluated (according to American Thoracic Society guidelines)6 as the absolute change in FEV1 and percentage change from initial FEV1, calculated as: post-FEV1 – pre-FEV1/pre-FEV1×100. Bronchial reversibility is defined as a drug-induced increase in FEV1 of ≥200 mL and ≥12% above baseline.
Statistical assessment
Results are presented as mean ± standard deviation. Statistical significance was at P<0.05. Descriptive group data were compared using the unpaired Student’s t-test and the Pearson chi-square test.
Ethical statement
The study was performed in accordance with the ethical principles of the Good Clinical Practice guidelines and with applicable local regulatory requirements. The protocol was approved by local ethics review boards. All patients read the patient information form about the study procedure, and written informed consents were obtained.
Results
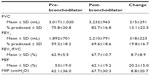
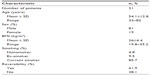
The baseline characteristics of patients are presented in Table 1. The female-to-male ratio was 4:17 and the mean age was 54.1±12.8 years. The mean body mass index (BMI) was 26.0±4.4 kg/m2. Eighty five point seven percent of patients were current smokers, 9.5% of patients were ex-smokers, and 4.8% of patients were nonsmokers. Pulmonary function test results and MIP values are presented in Table 2. The baseline spirometry results were: mean FVC, 3,017±1,020 mL and 75.8%±20.8%; mean FEV1, 1,892±701 mL and 59.2%±18.2%; FEV1/FVC, 62.9%±5.5%; peak expiratory flow (PEF), 53%±19%. The pre-bronchodilator mean MIP value was 62.1±36.9 cmH2O. A positive reversibility test with salbutamol was found in 61.9% of patients. The absolute change and percentage change in FEV1 were 318±223 mL and 19.8%±16.7%, respectively. The MIP value was increased by 5.5 cmH2O (8.8%) and was 67.7±30.3 cmH2O after bronchodilator. The relationship between the changes in MIP with age, FEV1, BMI, and reversibility are shown in Table 3. There was no significant relationship between age, FEV1, reversibility, and change in MIP with bronchodilator. However, the increase in MIP with bronchodilator was higher in patients with low BMI (<25 kg/m2) (P<0.05).
  | Table 1 Characteristics of patients |
Discussion
According to our knowledge, this is the first study to have investigated the relationship between change in MIP and bronchodilator. Therefore, this discussion is limited. MIP and MEP values were lower in patients with severe obstruction, compared with healthy subjects. MIP decreased also in patients with mild and moderate functional impairment. As with vital capacity, a high MIP (say, >80 cmH2O) is of great value in excluding clinically-important inspiratory muscle weakness.6 The factors contributing to respiratory muscle weakness in patients with COPD are: a) malnutrition-related biochemical, anatomical, and physiological changes; b) muscular atrophy; c) steroid-induced myopathy; d) pulmonary hyperinflation with increased residual volume; e) reduced blood flow to the respiratory muscles.7–13 Terzano et al reported a mean MIP value of 77±28 cmH2O in COPD patients, with mean FVC, FEV1, and PEF results as 77%±18%, 65%±22%, and 70%±24%, respectively.1 In our study, the mean MIP value (62.1±36 cmH2O) was lower than Terzano’s study, because the mean FEV1 was lower (59.2%±18.2%). Akkoca et al reported mean MIP values as 43.6±4.5 cmH2O (in patients with FEV1 ≤49%) and 67.7±5.5 cmH2O (in patients with FEV1 ≥50%).14 These values are consistent with our results.
In the present study, the reversibility test was found to be positive in 61.9% of patients. The absolute change and percentage of change in FEV1 were 317±223 mL and 19.8%±16.7%, respectively. The MIP value was increased by 8.8% after bronchodilator. To our knowledge, there are no other such results in the literature. There is a need for similar studies with larger numbers of patients; our study may provide guidance.
The change in MIP with bronchodilator was not affected by age, FEV1, or reversibilty. But it was affected by BMI, and the increase in MIP with bronchodilator was higher in patients with low BMI (<25 kg/m2). Terzano et al showed a significant linear relationship between respiratory muscle pressure and height, as seen in our patients.1
In conclusion, when we perform the reversibility test with salbutamol in patients with airway obstruction, we noted a 13.1% increase in FVC, 19.8% increase in FEV1, 20.2% increase in PEF, and 8.8% increase in MIP. MIP increases with bronchodilator therapy, regardless of changes in lung function. The reversibilty test with salbutamol can be used to evaluate MIP changes.
Disclosure
The authors report no conflicts of interest in this work.
References
Terzano C, Ceccarelli D, Conti V, Graziani E, Ricci A, Petroianni A. Maximal respiratory static pressures in patients with different stages of COPD severity. Respir Res. 2008;9:8. | |
Black LF, Hyatt RE. Maximal respiratory pressure: normal values and relationship to age and sex. Am Rev Respis Dis. 1969;99(5):696–702. | |
Karvonen J, Saarelainen S, Nieminen MM. Measurement of respiratory muscle forces based on maximal inspiratory and expiratory pressures. Respiration. 1994;61(1):28–31. | |
Syabbalo N. Assessment of respiratory muscle function and strength. Postgrad Med J. 1998;74(870):208–215. | |
Ozkaya S, Dirican A, Tuna T. The effects of long-acting β2-agonists plus inhaled corticosteroids for early reversibility in patients with airway obstruction. J Thorac Dis. 2013;5(4):461–465. | |
Hamnegard C-H, Wragg S, Kyroussis D, Aquilina R, Moxham J, Green M. Portable measurement of maximum mouth pressures. Eur Respir J. 1994;7(2):398–401. | |
Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine, Vol. 159, Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease (1999), pp. S2–S40. | |
Rochester DF. Malnutrition and the respiratory muscles. Clin Chest Med. 1986;7(1):91–99. | |
Openbrier DR, Irwin MM, Rogers RM, et al. Nutritional status and lung function in patients with emphysema and chronic bronchitis. Chest. 1983;83(1):17–22. | |
Decramer M, Stas KJ. Corticosteroid-induced myopathy involving respiratory muscles in patients with chronic obstructive pulmonary disease or asthma. Am Rev Respir Dis. 1992;146(3):800–802. | |
Van Balkom RH, Zhan WZ, Prakash YS, Dekhuijzen PN, Sieck GC. Corticosteroid effects on isotonic contractile properties of rat diaphragm muscle. J Appl Physiol (1985). 1997;83(4):1062–1067. | |
Nishimura Y, Tsutsumi M, Nakata H, Tsunenari T, Maeda H, Yokoyama M. Relationship between respiratory muscle strength and lean body mass in men with COPD. Chest. 1995;107(5):1232–1236. | |
Heijdra YF, Dekhuijzen PN, van Herwaarden CL, Folgering HT. Effects of body position, hyperinflation, and blood gas tensions on maximal respiratory pressures in patients with chronic obstructive pulmonary disease. Thorax. 1994;49(5):453–458. | |
Akkoca O, Demir G, Saryal S, Karabiyikoglu G. [The effect of hyperinflation on respiratory muscles and breathing pattern in COPD]. Tuberk Toraks. 2003;51(3):244–252. Turkish. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.