Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
The prevalence and correlates of the positive Androgen Deficiency in the Aging Male (ADAM) questionnaire among psychiatric outpatients: a cross-sectional survey of 176 men in a general hospital in Taiwan
Authors Lee C , Chen Y, Jiang K, Chu C, Hsu S, Chen J, Chen CY
Received 11 October 2014
Accepted for publication 10 November 2014
Published 20 January 2015 Volume 2015:11 Pages 185—189
DOI https://doi.org/10.2147/NDT.S75701
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Chin-Pang Lee,1,2 Yu Chen,2–4 Kun-Hao Jiang,2,4,5 Chun-Lin Chu,1,2,4 Shih-Chieh Hsu,1,2,4 Jiun-Liang Chen,2,4,5 Ching-Yen Chen1,2,4
1Department of Psychiatry, Chang Gung Memorial Hospital, Linkou, Taiwan; 2Men’s Health Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan; 3Department of Urology, Chang Gung Memorial Hospital, Linkou, Taiwan; 4School of Medicine, Chang Gung University, Taoyuan; 5Department of Traditional Chinese Medicine, Chang Gung Memorial Hospital, Linkou, Taiwan
Introduction: The Androgen Deficiency in the Aging Male (ADAM) questionnaire is widely used to screen for late-onset hypogonadism. The positive response to the ADAM questionnaire (positive ADAM) has been associated with depression and poorer quality of life in a number of studies. It is unclear whether there is any value of the ADAM questionnaire in psychiatric populations. In this study, we aimed to determine the utility of the ADAM questionnaire in a convenient sample of male psychiatric outpatients.
Methods: One hundred and seventy-six men (mean age: 54.3 years; standard deviation: 10.7 years; range: 40–80 years) completed the ADAM questionnaire, the Hospital Anxiety and Depression Scale (HADS), and the Aging Males’ Symptoms (AMS) scale. Anxiety was defined as a HADS anxiety subscore ≥8; depression as a HADS depression subscore ≥8; and moderate/severe impairment of health-related quality of life (HQoL) as AMS ≥37. ADAM, anxiety, and depression was used to model the moderate/severe impairment of HQoL.
Results: One hundred and sixty-four (93%) men had positive ADAM. Positive ADAM was associated with a lower body mass index (P<0.05) and moderate/severe impairment of HQoL (P<0.001), but was not associated with anxiety or depression (P>0.05). Positive ADAM was associated with five symptoms of the AMS scale: “decline of one’s feeling of general well-being”, “depressive mood”, and three sexual symptoms. In regression analysis, positive ADAM was associated with increased risk of moderate/severe impairment of HQoL (unadjusted odds ratio 20.1, 95% confidence interval 3.77–372, P<0.01), which remained significant with covariates of anxiety and depression (adjusted odds ratio 15.6, 95% confidence interval 2.52–309, P<0.05).
Conclusion: The ADAM questionnaire can be used to screen the sexual symptoms but not depression/anxiety in male psychiatric outpatients. Positive ADAM may indicate moderate/severe impairment of HQoL.
Keywords: Aging Males’ Symptoms scale, anxiety, depression, health-related quality of life
Introduction
The Saint Louis University Androgen Deficiency in the Aging Male (ADAM) questionnaire is widely used to screen late-onset hypogonadism,1 it has a sensitivity of 72%–88%, and has a specificity of 20%–32% for androgen deficiency in Chinese populations.2,3 Due to its lack of specificity, it is not appropriate to be used as a surrogate to serum testosterone testing. However, self-tests on the ADAM questionnaire are available online. It is not uncommon in daily practice to see middle-aged or older male patients seek help for “andropause”, which is indicated by the ADAM questionnaire. Nonetheless, the positive response to the ADAM questionnaire (positive ADAM) has been associated with depression and poorer health-related quality of life in a number of studies.4–7 The samples of the aforementioned studies include community-dwelling men and urological outpatients. To our knowledge, the ADAM questionnaire has not been investigated in psychiatric populations.
Like women, men experience an age-related decline of physical and mental capcity.8 Aging male symptoms are of multifactorial origin, such as medical problems, mental disorders, testosterone levels, and androgen receptor polymorphism.9 A number of studies suggest that high aging male symptoms may be associated with depressive and anxiety disorders, alexithymia,9–11 and poor quality of life.12,13
In the present study, we aimed to determine the prevalence of positive ADAM among male psychiatric outpatients and to examine the association between positive ADAM and depression/anxiety as well as aging male symptoms, health-related quality of life (HQoL).
Methods
Participants
We reanalyzed the data from the validation study of the Chinese version of the Aging Males’ Symptoms (AMS) scale.14 In 2006, we conducted a cross-sectional survey in the psychiatric clinic of Chang Gung Memorial Hospital, Linkou, Taiwan. This study was approved by the local institutional review board of Chang Gung Memorial Hospital. We recruited a convenience sample of 176 men (mean age: 54.3 years; standard deviation: 10.7 years; range: 40–80 years). The inclusion criteria were: 1) age 40 years or above; 2) Taiwan resident; and 3) able to speak and understand Mandarin. The exclusion criteria were: 1) history of psychotic, bipolar, and cognitive disorders; 2) active substance use disorder; 3) blindness, language or communication disorders; and 4) receiving psychotropic agents. Data were collected by face-to-face interviews.
Demographics
We recorded demographic information such as patients’ age, their spouse’s age, education attainment, employment status, and marital status. Both height and weight of the participants were measured to derive the body mass index (BMI) as weight (in kg) divided by the square of height (in m).
Instruments
ADAM questionnaire
The ADAM questionnaire is a ten-item self-administered dichotomous scale.1 Its ten items include decreased libido; lack of energy; reduced strength and/or endurance; loss of height; decreased enjoyment of life; sadness/grumpiness; erectile dysfunction; deterioration of performance in sports and work; and falling asleep after dinner. A positive ADAM is defined as a “yes” to decreased libido or erectile dysfunction, or to any three other symptoms. The validated Chinese version has good internal consistency (Cronbach’s α =0.74) and test–retest reliability (Pearson’s correlation coefficient r=0.86).15
Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) is a 14-item polychotomous scale that is widely used for assessment of anxiety and depression among medical patients and the general population.16,17 Respondents rate each item in a four-point response category (0–3: none =0; mild =1; moderate =2; and severe =3), so the possible scores ranged from 0 to 21 for the anxiety subscale (HADS-A) and 0 to 21 for the depression subscale (HADS-D). HADS focuses on the affective rather than somatic symptoms of anxiety disorders and depression. Cronbach’s α for HADS-A varied from 0.68 to 0.93 (mean 0.83), and for HADS-D from 0.67 to 0.90 (mean 0.82).18 With a threshold of ≥8,16 the sensitivity and specificity of HADS-A and HADS-D for anxiety disorders and depression are in the range of 0.70–0.90.18 The area under a receiver operating characteristic curve of HADS for anxiety disorders and depression is in the range of 0.84–0.96.18 When compared with other questionnaires for anxiety and depression such as General Health Questionnaire and Beck Depression Inventory, the correlations to HADS-A and HADS-D are in the range of 0.60 to 0.80.18 We used the validated Chinese version of HADS19 in this study. We used a threshold of ≥8 of HADS-A and HADS-D for presence of clinically significant anxiety and depression, respectively.
AMS
The AMS scale is a 17-item polychotomous scale to measure the health-related quality of life among aging men.8 Respondents rate each item in a five-point response category (1–5: none =1; mild =2; moderate =3; severe =4; and extremely severe =5), so the possible scores ranges from 17 to 85 for the AMS scale. Its 17 items include decline of one’s feeling of general well-being; joint pain and muscular ache; excessive sweating; sleep problems; increased need for sleep; irritability; nervousness; anxiety; physical exhaustion; decrease in muscular strength; depressive mood; feelings of having passed one’s peak; decrease in beard growth; decrease in ability/frequency to perform sexually; decrease in the number of morning erections; and decrease in sexual desire/libido. The severity of symptoms according to the total score was classified as none/little (17–26), mild (27–36), moderate (37–49), and severe (50 or more). We used the Taiwanese version of the AMS scale14 in this study. The Cronbach’s α was 0.90, and the 3-week retest reliability was 0.72.14 We used a threshold of ≥37 for presence of moderate/severe impairment of HQoL.
Statistical analysis
All analyses were conducted in R version 3.1.1 with packages of Hmisc and stargazer (R Foundation for Statistical Computing, Vienna, Austria). We examined the demographic characteristics and responses to the HADS and AMS scales. We used two sample t-tests with equal variance for continuous or ordinal variables, and used Pearson’s χ2 test or Fisher’s exact test for categorical variables. For analysis of the 17 AMS items by the response to the ADAM questionnaire, we used Bonferroni correction to control the familywise error rate. We used logistic regression analysis to model the moderate/severe impairment of HQoL by the response to ADAM questionnaire, as well as by anxiety and depression. The P-values were two-tailed, and the α-level was set at 0.05.
Results
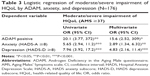
Of 176 men, 164 (93%) had positive ADAM. Table 1 shows the demographic characteristics as well as the responses to the HADS and AMS scales by response to the ADAM questionnaire. Patients with positive ADAM had a lower BMI and scored higher in HADS-A, HADS-D, and AMS than those with negative ADAM. Positive ADAM was not associated with anxiety or depression, but was associated with moderate/severe impairment of HQoL. Table 2 shows the results of two sample t-tests with equal variance of the 17 AMS items by the response to the ADAM questionnaire. With Bonferroni correction, positive ADAM was associated with five items of the AMS scale: AMS1 (decline of one’s feeling of general well-being), AMS11 (depressive mood), AMS15 (decrease in ability/frequency to perform sexually), AMS16 (decrease in the number of morning erections), and AMS17 (decrease in sexual desire/libido). Table 3 shows the results of logistic regression analysis of the moderate/severe impairment of HQoL by the response to ADAM questionnaire, as well as by anxiety and depression. Positive ADAM was associated with an unadjusted 20-fold risk and an adjusted 15-fold risk for moderate/severe impairment of HQoL, independent of anxiety and depression.
Discussion
In this study, we showed a 93.2% prevalence of positive ADAM among male psychiatric outpatients of age 40–80 years, which was comparable to73%–80% in two multicenter studies in Taiwan,2,3 87.8% in Hong Kong,20 77.6% in Saudi Arabia,21 and 81.3% in Chile.22 With regard to the low specificity of the ADAM questionnaire, our results support that the ADAM questionnaire would be of little value for screening late-onset hypogonadism, particularly in samples with high prevalence of psychological symptoms.22
We showed an association of positive ADAM and a lower BMI, echoing Pastuszak et al7 but Liu et al showed no such association.23 As we did not record other indicators of obesity such as waist circumference, the association of BMI and the response to the ADAM questionnaire requires further validation.
As was the case with Lee et al20 positive ADAM was associated with a higher score on the AMS and HADS scales, indicating more symptomatic and poorer health-related quality of life. However, positive ADAM was not associated with clinically significant anxiety or depression. The ADAM questionnaire might not be suitable for screening of anxiety or depressive disorders. By using the AMS scale, we showed that the symptomatology of positive ADAM was characterized by impaired well-being, depressed mood, and sexual complaints among psychiatric outpatients, consistent with the initial construct of the ADAM questionnaire.1 Although the ADAM questionnaire contains items of decreased enjoyment of life and sadness/grumpiness, which may be present in anxiety or depressive disorders, our results support that the ADAM questionnaire would measure different phenomena compared with the HADS. As the ADAM questionnaire has only ten dichotomous items and is easier to be completed than the AMS scale, it might be suitable for screening sexual symptoms among psychiatric outpatients.
We showed that positive ADAM was associated with moderate/severe impairment of HQoL independently of clinically significant anxiety and depression. As shown in our previous study,24 assessment of anxiety and depression by the AMS scale might be similar to assessment of anxiety and depression by the HADS. Clinicians would have to rule out anxiety and depressive disorders in patients with high AMS scores. In this respect, the ADAM questionnaire would help identify cases who have a poor health-related quality of life and need intervention in addition to treatment for anxiety and depression.20 In particular, clinicians should explore sexual symptoms among patients who report positive ADAM.
Several limitations of the present study should be recognized. Firstly, our results were cross-sectional and cannot indicate change over time or causality. Nonetheless, all three questionnaires used in our studies have good retest reliability.14,15,18 Secondly, our study was based on a convenience sample comprised of outpatients with neurotic complaints, which was of course bound to be highly selective, and hence limited generalization from our data. Our results also can not be applied to severely ill psychiatric patients, and it would not be appropriate to use questionnaires alone for assessment of severely ill patients. Thirdly, our data fall within the diagnostic heterogeneity of psychiatric disorders. We did not perform diagnostic interviews such as the Structured Clinical Interview for DSM-IV (SCID)25 to determine psychiatric diagnoses in the sample. Nonetheless, as the HADS has good sensivity and specificity for anxiety and depressive disorders,18 our results would not be considerably different whether using SCID or not. Furthermore, a dimensional approach of anxiety and depression has been suggested as more powerful and homogeneous than the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) category of anxiety and depression.26 Fourthly, we did not record confounding factors of depression and anxiety such as perceived stress and suicidal ideation, which might also explain the association between positive ADAM and minor anxiety and depressive symptoms. But the association between positive ADAM and moderate/severe impairment of HQoL was robust and independent of anxiety and depression. Hence, we considered that these confounding factors would not affect our results significantly. Lastly, we did not perform hormone and medical workups as physical illness might be responsible for affective and somatic symptoms. Nonetheless, the economic and technical constraints are common to clinical settings. Self-administered questionnaires are efficient tools and provide some insight in phenomenological investigation.
Conclusion
Positive ADAM was highly prevalent among psychiatric outpatients. The ADAM questionnaire can be used to screen sexual symptoms but not depression/anxiety in male psychiatric outpatients. Positive ADAM may indicate moderate/severe impairment of HQoL independent of anxiety and depression.
Disclosure
The authors report no conflicts of interest in this work.
References
Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49:1239–1242. | ||
Chueh KS, Huang SP, Lee YC, et al. The comparison of the aging male symptoms (AMS) scale and androgen deficiency in the aging male (ADAM) questionnaire to detect androgen deficiency in middle-aged men. J Androl. 2012;33:817–823. | ||
Lin YC, Hwang TI, Chiang HS, et al. Correlations of androgen deficiency with clinical symptoms in Taiwanese males. Int J Impot Res. 2006;18:343–347. | ||
Delhez M, Hansenne M, Luycks F, Legros JC. Que penser d’un test ADAM positif en l’absence d’hypogonadisme? [What about a positive test of ADAM in the absence of hypogonadism?] Ann Endocrinol (Paris). 2001;62:424. French. | ||
Tancredi A, Reginster JY, Schleich F, et al. Interest of the androgen deficiency in aging males (ADAM) questionnaire for the identification of hypogonadism in elderly community-dwelling male volunteers. Eur J Endocrinol. 2004;151:355–360. | ||
Beutel ME, Wiltink J, Hauck EW, et al. Correlations between hormones, physical, and affective parameters in aging urologic outpatients. Eur Urol. 2005;47:749–755. | ||
Pastuszak AW, Badhiwala N, Lipshultz LI, Khera M. Depression is correlated with the psychological and physical aspects of sexual dysfunction in men. Int J Impot Res. 2013;25:194–199. | ||
Heinemann LAJ, Zimmermann T, Vermeulen A, Thiel C, Hummel W. A new ‘aging males’ symptoms’ rating scale. Aging Male. 1999;2:105–114. | ||
Schneider G, Nienhaus K, Gromoll J, Heuft G, Nieschlag E, Zitzmann M. Aging males’ symptoms in relation to the genetically determined androgen receptor CAG polymorphism, sex hormone levels and sample membership. Psychoneuroendocrinology. 2010;35:578–587. | ||
Yoshida NM, Kumano H, Kuboki T. Does the Aging Males’ Symptoms scale assess major depressive disorder?: A pilot study. Maturitas. 2006;53:171–175. | ||
Honkalampi K, Lehto SM, Hintikka J, Koivumaa-Honkanen H, Niskanen L, Viinamäki H. Symptoms of depression and alexithymic burden in middle-aged men. Psychother Psychosom. 2010;79:259–261. | ||
Valenti G, Capone M, Forti G, et al. Inverse relationship between scores on the quality of life questionnaire SF-12 and on the Aging Males’ Symptoms scale in Italian men. Aging Male. 2008;11:77–82. | ||
Perchon LF, Pintarelli VL, Bezerra E, Thiel M, Dambros M. Quality of life in elderly men with aging symptoms and lower urinary tract symptoms (LUTS). Neurourol Urodyn. 2011;30:515–519. | ||
Chen CY, Wang WS, Liu CY, Lee SH. Reliability and validation of a Chinese version of the Aging Males’ Symptoms scale. Psychol Rep. 2007;101:27–38. | ||
Chu LW, Tam S, Kung AW, et al. A short version of the ADAM Questionnaire for androgen deficiency in Chinese men. J Gerontol A Biol Sci Med Sci. 2008;63:426–431. | ||
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. | ||
Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003;1:29. | ||
Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J Psychosom Res. 2002;52:69–77. | ||
Leung CM, Ho S, Kan CS, Hung CH, Chen CN. Evaluation of the Chinese version of the Hospital Anxiety and Depression Scale. A cross-cultural perspective. Int J Psychosom. 1993;40:29–34. | ||
Lee AM, Chu LW, Chong CS, et al. Relationship between symptoms of androgen deficiency and psychological factors and quality of life among Chinese men. Int J Androl. 2010;33:755–763. | ||
Rabah DM, Arafa MA. Validation of an Arabic ADAM questionnaire for androgen deficiency screening in the Arab community. Aging Male. 2009;12:95–99. | ||
Blümel JE, Chedraui P, Gili SA, Navarro A, Valenzuela K, Vallejo S. Is the Androgen Deficiency of Aging Men (ADAM) questionnaire useful for the screening of partial androgenic deficiency of aging men? Maturitas. 2009;63:365–368. | ||
Liu CC, Lee YC, Wang CJ, et al. The impact of androgen receptor CAG repeat polymorphism on andropausal symptoms in different serum testosterone levels. J Sex Med. 2012;9:2429–2437. | ||
Lee CP, Jiang JR, Chen Y, et al. The “Aging Males’ Symptoms” (AMS) Scale assesses depression and anxiety. Aging Male. 2013;16:97–101. | ||
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT). New York: Biometrics Research, New York State Psychiatric Institute, 2007. | ||
Wardenaar KJ, Vreeburg SA, van Veen T, et al. Dimensions of depression and anxiety and the hypothalamo-pituitary-adrenal axis. Biol Psychiatry. 2011;69:366–373. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.