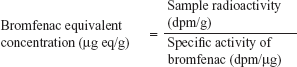Back to Journals » Clinical Ophthalmology » Volume 8
The ocular distribution of 14C-labeled bromfenac ophthalmic solution 0.07% in a rabbit model
Authors Baklayan G, Muñoz M
Received 22 April 2014
Accepted for publication 26 June 2014
Published 4 September 2014 Volume 2014:8 Pages 1717—1724
DOI https://doi.org/10.2147/OPTH.S66638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
George A Baklayan, Mauricio Muñoz
Bausch + Lomb, Irvine, CA, USA
Purpose: To evaluate the ocular distribution of an advanced formulation of bromfenac ophthalmic solution. Two studies were conducted in rabbits: 1) a 12-hour parallel-group study comparing the ocular distribution of 14C-bromfenac ophthalmic solution 0.07%, pH 7.8 with that of 14C-bromfenac ophthalmic solution 0.09%, pH 8.3, and 2) a 24-hour study evaluating the ocular distribution of 14C-bromfenac ophthalmic solution 0.07%, pH 7.8.
Methods: In the 12-hour study, rabbits were randomized to receive 50 µL of 14C-bromfenac 0.07%, pH 7.8 or 50 µL 14C-bromfenac 0.09%, pH 8.3 in one eye, whereas, in the 24-hour, study both eyes received 50 µL of 14C-bromfenac 0.07%, pH 7.8. Ocular tissues were collected at 1, 2, 4, 8, 12 (both studies) and 24 hours (second study only) following drug instillation, and tissue radioactivity was determined using liquid scintillation chromatography.
Results: Measureable levels of bromfenac were observed in all ocular tissues, with the exception of vitreous humor, regardless of formulation. In the 12-hour study, high concentrations of 14C-bromfenac were found in the sclera, followed by the iris/ciliary body, aqueous humor, choroid, retina, and lens. There was no significant difference between the bromfenac 0.07%, pH 7.8 and bromfenac 0.09%, pH 8.3 formulations in any 14C-bromfenac tissue levels at any time point, with the exception of in sclera at 2 hours post-instillation (0.451 µg eq/g versus 0.302 µg eq/g, respectively, P<0.001). There was also no significant difference in the total amount of 14C-bromfenac in the tissues evaluated following instillation of the two formulations. In the 24-hour study evaluating bromfenac 0.07%, pH 7.8 only, high concentrations of 14C-bromfenac were found 1 hour post-instillation in the cornea (2.402 µg eq/g) and conjunctiva (1.049 µg eq/g), two tissues not evaluated in the 12-hour study. The rank order of 14C-bromfenac levels in the other ocular tissues was the same as that observed in the 12-hour study, with measureable amounts of 14C-bromfenac detected through 24 hours in all tissues with the exception of vitreous humor.
Conclusion: Bromfenac ophthalmic solution 0.07%, pH 7.8 readily penetrated ocular tissues with levels similar to those of bromfenac ophthalmic solution 0.09%, pH 8.3.
Keywords: penetration, topical NSAIDs, pharmacokinetics, eye, pH
Introduction
Topical ophthalmic drugs are often reformulated for improved bioavailability and/or reduced dosing frequency. Most often this is accomplished by increasing the concentration of the active ingredient in the formulation. Another approach is to modify the vehicle without increasing the concentration.1 Recently, bromfenac ophthalmic solution was reformulated with a lower pH to allow for a decreased concentration of the active ingredient while maintaining high intraocular tissue exposure. In order to provide clinicians insight into the applicability of the modified bromfenac formulation in the management of various ocular disorders, studies were conducted to evaluate the ocular penetration of bromfenac in the modified formulation and compare it to a previously marketed reference formulation.
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are routinely used for treatment of inflammation and pain secondary to cataract surgery, and are sometimes preferred over topical corticosteroids to avoid steroid-associated adverse events such as elevated intraocular pressure and cataract formation.2–4 In the United States, bromfenac ophthalmic 0.09% solution is a topical NSAID indicated for the treatment of postoperative inflammation and reduction of ocular pain in patients who have undergone cataract extraction. The 0.09% formulation (or 0.1% when labeled as the salt form) has been shown to be as effective as betamethasone in minimizing inflammation following cataract surgery.5 In April 2013, Bausch + Lomb gained US Food and Drug Administration (FDA) approval for bromfenac 0.07% (Prolensa®; Bausch + Lomb, Irvine, CA, USA), a once-daily, advanced formulation of bromfenac ophthalmic solution.6 Prolensa® was reformulated from bromfenac 0.09% (Bromday®; Bausch + Lomb) to achieve similar ocular bioavailability with a lower concentration of active drug, thereby ensuring similar clinical efficacy to Bromday® but with reduced exposure of the surgically compromised ocular surface to the drug. In order to lower the concentration yet maintain the same degree of ocular penetration, the pH of the formulation was reduced from 8.3 (Bromday®) to 7.8 (Prolensa®). Bromfenac, like most NSAIDs, is a weakly acidic drug.7 Decreasing the pH of the formulation increases the unionized fraction of the drug, which, in turn, enhances ocular penetration.7
NSAIDs have anti-inflammatory properties mediated by the inhibition of the cyclooxygenase (COX) pathway and prostaglandin (PG) synthesis. PGs, which increase after cataract surgery, disrupt the blood–aqueous humor barrier and increase vascular permeability, vasodilation, and intraocular pressure, leading to ocular inflammation.8,9 Studies have shown that the COX-2 enzyme, considered the primary mediator of ocular inflammation,10 is upregulated in animal models of ocular inflammation and ocular injury.11,12
In order to reduce inflammation effectively, ophthalmic NSAIDs need to both penetrate the affected tissues and bind to the COX-2 enzyme. A study of the physiochemical properties of amfenac and its derivatives found that the addition of a substituent group to the aromatic ring of the molecule decreased, while the addition of a halogen group (Br~I>Cl>F) to the 4′-position of the aromatic ring increased anti-inflammatory potency.13 The presence of a bromine in the 4′-position, as in bromfenac, was found to improve in vitro and in vivo potency, absorption across the cornea, and penetration into ocular tissues.13–15 Bromfenac is a selective COX-2 inhibitor with an IC50 approximately 32 times more active against COX-2 than COX-1 (IC50 of 0.0066 μM and 0.210 μM, respectively),16 and it has been shown in an animal model to significantly prevent PGE2 production compared with nepafenac (P<0.05).17 Bromfenac is also highly lipophilic, with a ClogP (octanol/water partition coefficient) of 2.23, which is higher than that of amfenac (1.23), suggesting that bromfenac may have increased penetration into ocular tissues and potentially a more rapid onset of action.15 A pharmacokinetic study in patients undergoing cataract surgery showed that bromfenac was absorbed within 30 minutes following instillation of bromfenac ophthalmic solution 0.1%, with a peak aqueous humor concentration at 2.5 to 3 hours after instillation.18 In addition, a previous animal study demonstrated that bromfenac 0.09%, pH 8.3 readily penetrates ocular tissues.19
The objective of the current two studies was to evaluate the ocular distribution of the new modified formulation of bromfenac ophthalmic solution. The first study evaluated the 12-hour ocular distribution profile of 14C-bromfenac ophthalmic solution 0.07%, pH 7.8 compared with 14C-bromfenac ophthalmic solution 0.09%, pH 8.3, while the second study evaluated the 24-hour ocular distribution of 14C-bromfenac ophthalmic solution 0.07%, pH 7.8 to confirm sustained ocular penetration with once-daily dosing.
Methods
Two separate preclinical studies were performed at the Biological Test Center ([BTC] Irvine, CA, USA) using similar protocols. Each study complied with the animal welfare policies of the BTC, was approved by the Institutional Animal Care and Use Committee (IACUC), and conformed to the ARVO (Association for Research in Vision and Ophthalmology) “Statement on the Use of Animals in Ophthalmic and Visual Research.”
Test article
The 14C-bromfenac was manufactured by Daiichi Pure Chemicals Co., Ltd., and provided by Senju Pharmaceuticals Co., Ltd., (Osaka, Japan). It was determined to be 98.3% pure by high-performance liquid chromatography (HPLC). The radiolabeled carbon atom was that of the carbonyl within the bromobenzoyl group of bromfenac (Figure 1), a stable position within the molecule.
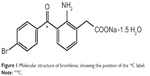  | Figure 1 Molecular structure of bromfenac showing the position of the 14C label. |
Dosing solutions were prepared by adding 14C-bromfenac to diluent to reach a concentration of 0.07%, pH 7.8 or 0.09%, pH 8.3. The resulting 14C-bromfenac 0.07%, pH 7.8 and the 0.09%, pH 8.3 formulations used in the studies were identical to the marketed Prolensa® and Bromday® formulations, respectively, with the exception of the inclusion of 14C-bromfenac. Following dosing, dosing solutions were reanalyzed by HPLC to confirm radioactive purity; all samples were determined to be 95% to 99% pure.
Animals
In both studies, female New Zealand White rabbits were obtained from The Rabbit Source (Ramona, CA, USA). Animals were at least 12 weeks of age and weighed 1.9 to 3.6 kg at the time of dosing. Animals were housed in individual cages and identified with ear tags and cage cards.
Pretreatment examination and dosing procedures
Prior to placement in the study, each animal underwent a pretreatment ophthalmic examination as well as a slit-lamp examination. Criteria for acceptance into the study were severity scores of ≤1 (on 0–3 or 0–4 scales) for conjunctival congestion and swelling, and the absence of any other slit-lamp signs. Prior to dosing, animals were weighed and randomly assigned to a study group. For the 12-hour study, animals were divided into groups receiving either 14C-bromfenac 0.07%, pH 7.8 or 14C-bromfenac 0.09%, pH 8.3 for collection of ocular tissues at one of five different time points (1, 2, 4, 8, and 12 hours). For the 24-hour study, animals were divided into groups receiving 14C-bromfenac 0.07%, pH 7.8 for collection of ocular tissue at one of six different time points (1, 2, 4, 8, 12, and 24 hours). There was no comparator arm in the 24-hour study.
On day 1 of each study, 50 μL of 14C-bromfenac dosing solution was administered into the conjunctival sac of each animal using a calibrated digital pipette, and the time of dose administration was noted. The eyelids were held closed post-dose for 5–10 seconds. Actual dose in mg and μCi were used in all subsequent calculations.
In the 12-hour parallel-group study, animals received either 14C-bromfenac 0.07%, pH 7.8 or 14C-bromfenac 0.09%, pH 8.3 in one eye. The specific activity of bromfenac in these formulations was 528,494 dpm/μg (or 0.23 μCi/μg) and 359,952 dpm/μg (or 0.16 μCi/μg), respectively. Three rabbit eyes were dosed per treatment per time point for a total of 15 eyes dosed for each test agent. Animals were euthanized by intravenous injection of a commercial euthanasia solution per the standard operating procedure of the animal facility (BTC) at predetermined time points (1 hour [±5 minutes], 2 hours [±15 minutes], 4 hours [±15 minutes], 8 hours [±15 minutes], or 12 hours [±15 minutes]) after instillation of test agent, and ocular tissues were immediately harvested. In the 24-hour study, animals were administered 14C-bromfenac 0.07%, pH 7.8 only (specific activity of 411,032 dpm/μg or 0.18 μCi/μg). Both eyes from three rabbits were dosed per time point over a 24-hour period for a total of six eyes dosed per time point. Animals were euthanized as described above at 1 hour (±5 minutes), 2 hours (±15 minutes), 4 hours (±15 minutes), 8 hours (±15 minutes), 12 hours (±15 minutes), or 24 hours (±15 minutes) after administration of test agent, and ocular tissues harvested.
In both studies, animals were observed for morbidity and mortality throughout the study.
Sample processing and radioactivity measurements
Tissues harvested included the aqueous humor, vitreous humor, iris/ciliary body, lens, retina, choroid, sclera (both studies), and cornea and conjunctiva (24-hour study only). While the 12-hour study focused on the tissues most relevant to treating postoperative inflammation, the 24-hour study, conducted later, was patterned from a previously developed 24-hour drug exposure protocol19 and focused on all ocular tissues and therefore also included the cornea and conjunctiva. Immediately following euthanasia, the aqueous humor was collected from the eye and frozen at −20°C if not immediately analyzed. The globe was then enucleated, snap-frozen in liquid nitrogen, and stored at ≤−70°C until dissection.
Iris/ciliary body, lens, retina, choroid, sclera (both studies), and cornea and conjunctiva (24-hour study only) were dissected from each eye, weighed into combustion cones, and combusted. Combusted samples were trapped in 14C-Cocktail (RJ Harvey, Hillsdale, NJ, USA) in liquid scintillation chromatography (LSC) vials and the amount of radioactivity was determined by LSC. To determine the amount of radioactivity in aqueous humor and vitreous humor, duplicate aliquots of each aqueous humor sample (25 μL) and homogenized vitreous humor sample (100 μL) were transferred to LSC vials and the amount of radioactivity determined following the addition of 10 mL of Insta-Gel (Perkin-Elmer Life and Analytical Sciences, Inc., Waltham, MA, USA).
Radioactivity measurements were made using a Beckman Liquid Scintillation Spectrometer (Beckman Coulter Inc., Fullerton, CA, USA). Counting time was to a statistical accuracy of ±2% or a maximum of 10 minutes, whichever came first. Background noise was automatically subtracted from each measurement and counts per minute were automatically converted to disintegrations per minute.
Statistical analysis
To determine the specific activity (dpm/μg) of 14C-bromfenac, the following formula was used:
| (1) |
To determine bromfenac-equivalent concentration of dosed 14C-bromfenac, the following formula was used:
| (2) |
Mean and standard deviation of each set of samples were used to characterize the data. Although the sample size (N=3 eyes per treatment per time period) in the 12-hour study was not powered to find a specific difference, Student’s t-tests were used to compare the groups.
Results
12-hour pharmacokinetic study
Following the administration of either 14C-bromfenac 0.07%, pH 7.8 or 14C-bromfenac 0.09%, pH 8.3, concentrations of bromfenac ranged from 0.000 to 0.595 μg eq/g depending on the tissue. The highest 14C-bromfenac levels were found in the sclera, followed by the iris/ciliary body, aqueous humor, choroid, retina, and lens (Table 1, Figures 1 and 2). The timing of peak concentrations of radiolabeled bromfenac varied among the tissues, with peak levels being reached at 1 hour in the sclera and retina and at 2 hours in the aqueous humor, iris/ciliary body, lens, and choroid. There was a significant difference between the formulations at the 2-hour time point for scleral 14C-bromfenac levels, with higher levels in the animals randomized to the bromfenac 0.07%, pH 7.8 treatment group (P<0.001). However, there were no other between-treatment differences at any other time points in any tissue. The total amount (ie, sum) of 14C-bromfenac in all ocular tissues evaluated was directionally higher in the 14C-bromfenac 0.07%, pH 7.8 group compared with the 14C-bromfenac 0.09%, pH 8.3 group in four out of five time points, but the differences were not statistically significant.
  | Figure 2 Ocular distribution of 14C-bromfenac in rabbits over 12 hours following topical administration of 14C-bromfenac 0.07%, pH 7.8 (A) or 14C-bromfenac 0.09%, pH 8.3 (B). |
24-hour pharmacokinetic study
After administration of 14C-bromfenac 0.07%, pH 7.8, 14C-bromfenac was detected in all tissues of the eye over a 24-hour period, with the exception of vitreous humor. As expected, the highest concentrations were seen in the cornea, followed by conjunctiva and sclera (Table 2 and Figure 3). The concentrations in all ocular tissues decreased to varying degrees over the 24-hour study period, with the exception of the lens, which exhibited little change from the 1-hour time point.
  | Table 2 Concentration of 14C-bromfenac in ocular tissues over 24 hours following topical administration of 14C-bromfenac 0.07%, pH 7.8 |
  | Figure 3 Ocular distribution of 14C-bromfenac in rabbits over 24 hours following topical administration of 14C-bromfenac 0.07%, pH 7.8. |
No adverse events were observed in either study.
Discussion
Ocular inflammation following surgery arises from a complex series of intracellular signaling events involving COX-1 and COX-2 enzymes. In particular, activation of the COX-2 signaling pathway leads to the production of PGs and thromboxane A2, which ultimately alters vascular permeability by changing the function of vascular endothelial cells.8 Topical NSAIDs are a class of anti-inflammatory drugs that have been used following cataract and other ocular surgeries, as well as in ocular disorders affecting the posterior chamber of the eye, due to their ability to inhibit the COX signaling pathways, thereby decreasing inflammation. In order for any topical NSAID to achieve its desired effects, the drug must penetrate the tissues of the eye, target the induced COX enzymes, and remain sustained within the tissue long enough to prevent or reduce PG effects. Once-daily dosing reduces the need for repeated applications and potentially improves patient adherence.
Bromfenac topical ophthalmic solution has been shown to quickly permeate the tissues of the eye, maintain measurable concentrations for up to 24 hours, and effectively inhibit the COX-2 signaling pathway, making it an effective NSAID in the treatment of ocular inflammation.9 Several studies have compared bromfenac with other topical NSAIDs for the ability to inhibit COX-2 activity and hence inhibit inflammation. Yanni et al compared the IC50 values of bromfenac, diclofenac, and amfenac for purified rabbit COX-2 and found that the inhibitory effect of bromfenac on COX-2 was 3.7 times greater than that of diclofenac and 6.5 times greater than that of amfenac.14 An additional study comparing the IC50 of bromfenac to amfenac, diclofenac, and ketorolac using recombinant human COX-2 demonstrated that bromfenac had the greatest activity against human COX-2, followed by amfenac, ketorolac, and diclofenac.20
An advanced formulation of bromfenac ophthalmic solution was recently developed which contains a lower concentration of bromfenac at a lower pH than previous formulations. It was hypothesized that reducing the pH from 8.3 from 7.8 would allow for a lower-concentration formulation to provide comparable penetration into ocular tissues as the reference product, bromfenac 0.09%, which has had considerable marketplace success. The 12- and 24-hour ocular distribution studies presented here demonstrate that bromfenac 0.07%, pH 7.8 was able to penetrate various ocular tissues and remain in those tissues at measurable levels for up to 24 hours. Even at a lower concentration, the bromfenac 0.07%, pH 7.8 solution exhibited similar, and in the case of scleral tissue, increased, penetration of ocular tissues studied, when compared with bromfenac 0.09%, pH 8.3 solution. This is likely due to the difference in pH between the solutions; lowering the pH increases the unionized fraction of drug, which can lead to enhanced corneal permeability.7 Additionally, the reduction in pH to a more physiologic level could reduce any potential for discomfort and irritation upon instillation. Although we used a healthy eye model in these studies, ocular penetration is likely to be higher in inflamed eyes due to weakened barriers from the underlying pathological condition.21 However, any differences between formulations is expected to be the same in an inflamed eye model.
Of note, if one uses an estimate of 1 mL for the volume of a gram of tissue, it follows that ocular tissue levels of bromfenac following topical administration of bromfenac ophthalmic solution 0.07%, pH 7.8 were sustained at concentrations above the reported IC50 of bromfenac for the COX-2 enzyme (0.0066 μM or 2.5 pg/mL) in all tissues evaluated for the duration of each study, with the exception of vitreous humor. That negligible levels of radiolabeled bromfenac were found in the vitreous humor following dosing with bromfenac in the 12-hour study as well as the 24-hour study independent of formulation was not surprising and was consistent with previous findings.19 As a highly lipophilic drug, bromfenac has a more rapid drug-transit time through tissues and/or choroidal blood flow to posterior-segment tissues, and is not expected to concentrate appreciably in the vitreous humor.
Previous studies of 14C-bromfenac ophthalmic solution 0.09%, pH 8.3 in rabbits have demonstrated that this solution rapidly achieved measurable levels in all major ocular tissues at or before 2 hours and detectable levels of bromfenac were sustained for up to 24 hours following a single ophthalmic dose.19 Similar to the results of the 24-hour study with bromfenac 0.07%, pH 7.8, the highest concentrations of radiolabeled bromfenac were detected in the cornea, conjunctiva, and sclera.19 Additional studies in human subjects undergoing cataract surgery have shown that a single dose of bromfenac ophthalmic solution 0.1% led to rapid absorption of the drug within 30 minutes of treatment and therapeutic levels (ie, above IC50 for bromfenac) were maintained within the ocular tissue for up to 12 hours.18
The ability of ophthalmic NSAIDs to inhibit PG synthesis suggests that they may be effective for ocular disorders affecting posterior tissues, such as cystoid macular edema (CME).22–25 While the use of NSAIDs in the prevention and treatment of CME is not approved by the US FDA, various studies evaluating the efficacy of bromfenac 0.9% ophthalmic solution in the management of CME26–30 and choroidal neovascularization associated with age-related macular degeneration31–33 have shown a potential benefit of bromfenac in the management of these disorders. The results of these 12- and 24-hour ocular distribution studies suggest that the ability of bromfenac to quickly and effectively penetrate ocular tissues, including posterior tissues, may support its use in treating other ocular inflammatory diseases, including CME.
Conclusion
As a new, advanced formulation of bromfenac ophthalmic solution, developed for once-daily dosing with a lower concentration and lower pH than previous formulations, it was important to evaluate the ocular distribution of 14C-bromfenac ophthalmic solution 0.07%, pH 7.8 up to 24 hours post-administration and to compare its ocular distribution with that of 14C-bromfenac ophthalmic solution 0.09%, pH 8.3. The pH of bromfenac ophthalmic solution 0.07% (Prolensa®) was reduced to 7.8, thereby increasing the ratio of non-ionized to ionized fraction and allowing for a lower concentration of active ingredient, while maintaining the ability to rapidly penetrate ocular tissues and remain in relevant ocular tissues for up to 24 hours. While these studies on the ocular distribution of bromfenac ophthalmic solution 0.07% were conducted in rabbits, the results suggest that the new formulation has the potential to be an effective agent for the treatment of inflammation in a number of ocular inflammatory diseases. Further clinical explorations into the use of bromfenac solution 0.07% in these settings will provide insight into the full range of therapeutic possibilities for bromfenac ophthalmic solution.
Acknowledgments
The authors wish to express our appreciation for the assistance of Chantelle Rein-Smith, PhD and Natalie Her, PhD of Whitsell Innovations Inc., for manuscript preparation and for the assistance of Linda Wang, PharmD, University of Southern California fellow training at Bausch + Lomb, for editorial assistance and data review/verification. This study was presented in part at the 85th Annual Meeting of the Association for Research in Vision and Ophthalmology, Inc.; May 5–9, 2013; Seattle, WA, USA.
Disclosure
George A Baklayan is an employee of Bausch + Lomb. Mauricio Muñoz was an employee of Bausch + Lomb at the time the manuscript was developed. Study support was provided by ISTA Pharmaceuticals, Inc., (Irvine, CA, USA). The sponsor participated in the design of the study, data collection, data management, data analysis, interpretation of the data, and preparation, review, and approval of the manuscript. Bausch + Lomb acquired ISTA Pharmaceuticals in June 2012. All bromfenac products are now marketed under the Bausch + Lomb trademark, unless otherwise specified. The preclinical trial discussed in this manuscript was conducted by ISTA Pharmaceuticals, Inc., and all rights were transferred to Bausch + Lomb upon completion of the acquisition. The authors report no other conflicts of interest in this work.
References
Bucolo C, Melilli B, Piazza C, Zurria M, Drago F. Ocular pharmacokinetics profile of different indomethacin topical formulations. J Ocul Pharmacol Ther. 2011;27(6):571–576. | ||
Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin. 1992;10(3):505–512. | ||
Becker B. The side effects of corticosteroids. Invest Ophthalmol. 1964; 3:492–497. | ||
McGhee CN. Pharmacokinetics of ophthalmic corticosteroids. Br J Ophthalmol. 1992;76:681–684. | ||
Miyanaga M, Miyai T, Nejima R, Maruyama Y, Miyata K, Kato S. Effect of bromfenac ophthalmic solution on ocular inflammation following cataract surgery. Acta Ophthalmol. 2009;87(3):300–305. | ||
Prolensa® [package insert]. Tampa, FL: Bausch + Lomb; 2013. | ||
Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008;10(2):229–241. | ||
Guex-Crosier Y. [Non-steroidal anti-inflammatory drugs and ocular inflammation]. Klin Monbl Augenheilkd. 2001;218(5):305–308. French. | ||
Gluud BS, Jensen OL, Krogh E, Birgens HS. Prostaglandin E2 level in tears during postoperative inflammation of the eye. Acta Ophthalmol (Copenh). 1985;63(4):375–379. | ||
Oka T, Shearer T, Azuma M. Involvement of cyclooxygenase-2 in rat models of conjunctivitis. Curr Eye Res. 2004;29(1):27–34. | ||
Miyamoto T, Saika S, Okada Y, et al. Expression of cyclooxygenase-2 in corneal cells after photorefractive keratectomy and laser in situ keratomileusis in rabbits. J Cataract Refract Surg. 2004;30(12):2612–2617. | ||
Radi ZA, Render JA. The pathophysiologic role of cyclo-oxygenases in the eye. J Ocul Pharmacol Ther. 2008;24(2):141–151. | ||
Walsh DA, Moran HW, Shamblee DA, et al. Antiinflammatory agents. 3. Synthesis and pharmacological evaluation of 2-amino-3-benzoylphenylacetic acid and analogues. J Med Chem. 1984;27(11):1379–1388. | ||
Yanni JM, Graff G, Hellberg MR, inventors; Alcon Laboratories Inc., assignee. Adminstering 3-benzoylphenylacetamide derivatives. United States patent US5475034. 1995 Dec 12. | ||
Ruiz J, López M, Milà J, Lozoya E, Lozano JJ, Pouplana R. QSAR and conformational analysis of the antiinflammatory agent amfenac and analogues. J Comput Aided Mol Des. 1993;7(2):183–198. | ||
Waterbury LD, Silliman D, Jolas T. Comparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodium. Curr Med Res Opin. 2006;22(6): 1133–1140. | ||
Bucolo C, Marrazzo G, Platania CB, Romano GL, Drago F, Salomone S. Effects of topical indomethacin, bromfenac and nepafenac on lipopolysaccharide-induced ocular inflammation. J Pharm Pharmacol. 2014;66(7):954–960. | ||
Miyake K, Ogawa T, Tajika T, Gow JA, McNamara TR. Ocular pharmacokinetics of a single dose of bromfenac sodium ophthalmic solution 0.1% in human aqueous humor. J Ocul Pharmacol Ther. 2008; 24(6):573–578. | ||
Baklayan GA, Patterson HM, Song CK, Gow JA, McNamara TR. 24-hour evaluation of the ocular distribution of (14)C-labeled bromfenac following topical instillation into the eyes of New Zealand White rabbits. J Ocul Pharmacol Ther. 2008;24(4):392–398. | ||
Kida T, Ogawa T, McNamara TR, Song CK, Gow JA. Evaluation of the human Cox-2 inhibition of amfenac, bromfenac, diclofenac, and ketorolac. Proceedings of the Annual American Society of Cataract and Refractive Surgery Conference; April 27 – May 2, 2007; San Diego, CA. | ||
Ueda K, Ohtori A, Tojo K. Effects of pathological conditions on ocular pharmacokinetics of antimicrobial drugs. Chem Pharm Bull (Tokyo). 2010;58(10):1301–1305. | ||
Cho H, Mozayan A. New look at ocular inflammation control – powerful and fast-acting twice-daily bromfenac for a novel standard in the treatment of inflammation. European Ophthalmic Review. 2011;5(1):20–26. | ||
O’Brien TP. Emerging guidelines for the use of NSAID therapy to optimize cataract surgery patient care. Curr Med Res Opin. 2005;21(7): 1131–1137. | ||
Jones J, Francis P. Ophthalmic utility of topical bromfenac, a twice-daily nonsteroidal anti-inflammatory agent. Expert Opin Pharmacother. 2009;10(14):2379–2385. | ||
Carreño E, Portero A, Galarreta DJ, Herreras JM. Update on twice-daily bromfenac sodium sesquihydrate to treat postoperative ocular inflammation following cataract extraction. Clin Ophthalmol. 2012;6: 637–644. | ||
Cable M. Comparison of bromfenac 0.09% QD to nepafenac 0.1% TID after cataract surgery: pilot evaluation of visual acuity, macular volume, and retinal thickness at a single site. Clin Ophthalmol. 2012;6: 997–1004. | ||
Warren KA, Bahrani H, Fox JE. NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. Retina. 2010;30:260–266. | ||
Radwan AE, Arcinue CA, Yang P, Artornsombudh P, Abu Al-Fadl EM, Foster CS. Bromfenac alone or with single intravitreal injection of bevacizumab or triamcinolone acetonide for treatment of uveitic macular edema. Graefes Arch Clin Exp Ophthalmol. 2013;251:1801–1806. | ||
Endo N, Kato S, Haruyama K, Shoji M, Kitano S. Efficacy of bromfenac sodium ophthalmic solution in preventing cystoid macular oedema after cataract surgery in patients with diabetes. Acta Ophthalmol. 2010;88(8): 896–900. | ||
Wang QW, Yao K, Xu W, et al. Bromfenac sodium 0.1%, fluorometholone 0.1% and dexamethasone 0.1% for control of ocular inflammation and prevention of cystoid macular edema after phacoemulsification. Ophthalmologica. 2013;229(4):187–194. | ||
Gomi F, Sawa M, Tsujikawa M, Nishida K. Topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degeneration. Retina. 2012;32:1804–1810. | ||
Flaxel C, Schain MB, Hamon SC, Francis PJ. Prospective randomized controlled trial of combination ranibizumab (Lucentis) and bromfenac (Xibrom) for neovascular age-related macular degeneration: a pilot study. Retina. 2012;32:417–423. | ||
Zweifel SA, Engelbert M, Khan S, Freund KB. Retrospective review of the efficacy of topical bromfenac (0.09%) as an adjunctive therapy for patients with neovascular age-related macular degeneration. Retina. 2009;29:1527–1531. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.