Back to Journals » Veterinary Medicine: Research and Reports » Volume 6
The etiology of digital dermatitis in ruminants: recent perspectives
Authors Wilson-Welder J, Alt D, Nally J
Received 27 November 2014
Accepted for publication 25 February 2015
Published 4 May 2015 Volume 2015:6 Pages 155—164
DOI https://doi.org/10.2147/VMRR.S62072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey MB Musser
Video abstract presented by Jarlath E Nally
Views: 4756
Jennifer H Wilson-Welder, David P Alt, Jarlath E Nally
Bacterial Diseases of Livestock Research Unit, National Animal Disease Center, Agricultural Research Service, United States Department of Agriculture, Ames, IA, USA
Abstract: Digital dermatitis (DD) is a multifactorial polymicrobial infectious disease originally described in dairy cattle, but is increasingly recognized in beef cattle, sheep, and more recently, elk and goats. Clinical bovine lesions typically appear on the plantar surface of the hind foot from the interdigital space and heel bulb to the accessory digits, with a predilection for skin–horn junctions. Lesions present as a painful ulcerative acute or chronic inflammatory process with differing degrees of severity. This variability reflects disease progression and results in a number of different clinical descriptions with overlapping pathologies that ultimately have a related bacterial etiology. The goal of this review article is to provide a concise overview of our current understanding on digital dermatitis disease to facilitate clinical recognition, our current understanding on the causative agents, and recent advances in our understanding of disease transmission.
Keywords: Digital dermatitis, treponemes, lameness, ruminant
Clinical presentation of digital dermatitis (DD) in ruminants
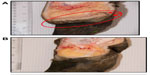
A typical active lesion associated with bovine digital dermatitis (BDD) is found on the plantar surface of the hind foot of a dairy cow that presents as a circumscribed moist ulcerative erosive mass along the coronary band or interdigital space (Figure 1A).1 Lameness is likely, as lesions are painful upon palpation and prone to bleeding when touched. Histologically, there is a loss of stratum corneum, epidermal hyperplasia, and reactive inflammation. Whilst the precise etiology of such lesions is not yet clear, it is apparent that treponemes are the major pathogenic bacteria detected in a series of related lesions associated with the bovine hoof.2 Further, it is increasingly apparent that such lesions are not restricted to bovines, and that treponemes (as well as other bacterial agents) are associated with a similar range of lesions that are increasingly observed in other ruminant species including sheep, elk and goats.3–5 Given that those treponemes associated with bovine digital dermatitis are genetically similar to those observed in contagious ovine digital dermatitis, hoof disease in elk, and severe lameness in goats, which occupy the same anatomical site, these diseases are not mutually exclusive. Collectively, they provide a greater insight to understand the range of clinical manifestations of digital dermatitis in large and small ruminants, as well as pathogenic mechanisms of infection that will facilitate an improved understanding of disease transmission, treatment, and prevention.
In 1974, Cheli and Mortellaro6 described a bovine digital dermatitis in Italy that affected 60%–70% of cows. A similar disease of the bovine hoof was reported shortly thereafter by Rebhun et al,7 in the US; interdigital papillomatosis was characterized by circular, edematous proliferative masses, and interdigital growths that caused severe lameness in approximately 70% of cows. The short disease transmission period was indicative of an infectious agent but preparation of an autogenous formalinized vaccine created from papillomas of the herd did not alter disease nor prevent recurrence. Whilst histopathology suggested that papillomas were of viral origin, no virus was identified by culture or electron microscopy.7 By mid-1980, similar clinical signs were routinely recognized on dairy farms throughout Europe and the US, and referred to by a range of names including digital dermatitis, interdigital dermatitis, interdigital papillomas, Mortellaro’s disease, hairy heel warts, and strawberry foot. Digital dermatitis and interdigital dermatitis are suggested to be the same disease, differing only by location of lesion.8 Digital dermatitis is a global disease that is estimated to cost the US $190 million per annum due to lameness associated with decreased milk yield.9
Although digital dermatitis was initially described as causing acute lameness, further studies have demonstrated that lesions develop through different stages which have been characterized grossly.10–13 At the macroscopic level, a qualitative classification system has been developed to identify the different levels of BDD lesion progression;14 Class I (M1) refers to the early stage of digital dermatitis which is a small circumscribed granulomatous area that is moist, ragged, mottled red-gray, 0.5–2 cm in diameter, and lies at the epithelial surface or up to 2 mm underneath it. Class II (M2) refers to the classical ulceration close to the coronary band, >2 cm in diameter, with granulomatous tissue where the lesion lies more than 2 mm underneath the epithelial layer. Class III (M3) refers to the healing process of the M2 lesion which is covered by a scab. Class IV (M4) lesions may be observed as the disease becomes endemic in herds and presents as an alteration of the skin close to the coronary band. Class IV (M4) refers to a hyperkeratotic lesion with a proliferative aspect varying in appearance from papilliform to mass-like projections. M4 has been further subdivided to include M4.1 that recognizes M4 with a small active painful M1 focus (Figure 1B).15 The early stages of lesion development (M1) have also been further subdivided to recognize the transition from normal skin (stage 0) to initial onset (stage 1) and developing lesions (stage 2). Stage 2 is further classified as a “type A” lesion if presenting in the interdigital space and has a more ulcerated appearance in comparison to a “type B” lesion which develops more diffusely across the heel with a thickened, crusted appearance.11 The morphological characteristics of lesions are not always easy to distinguish and can be interrelated or concurrent and the etiopathogenesis of the conditions may overlap. Whilst a specific lesion may be painful upon palpation, not all affected animals will be clinically lame.
Histopathologically, lesions are classified as bovine digital dermatitis if they comprise 1) a circumscribed plaque of eroded acanthotic epidermis attended by parakeratotic papillomatous proliferation colonized by spirochetes (treponemes), 2) loss of stratum granulosum, 3) invasion of stratum spinosum by spirochetes and 4) infiltration of neutrophils, plasma cells, lymphocytes, and eosinophils in dermis.1,16 Histological activity can be focal, segmental, or continuous. Early stage lesions tend to be described as hyperkeratotic, acanthotic with surface hemorrhage and erythrocytic crusts, whereas developing and end-stage lesions have segmental localized necrotizing to necrosuppurative epidermitis with individual cell necrosis, ballooning degeneration of epithelial cells, necrotizing vasculitis, and intralesional bacteria including spirochetes. There is no clear indication of the rate of development of lesions; transition between disease states is reported to range from as few as 12 days to as long as 135 days with an upper limit of almost 2 years.11,17,18 However, such variation likely reflects how often and thoroughly that lesions are inspected.
Heel horn erosion, also known as slurry-heel or heel necrosis, is defined as “an irregular loss of bulbar horn” and associated with an unhygienic environment since manure and urine induce structural breakdown of the horn tissue. Dermatitis at the skin-horn junction can result in changes in growth of the horn leading to deterioration. There is a strong association between the presence of heel horn erosion and digital dermatitis, and spirochetes (treponemes).2 Heel horn erosion has other environmental causes and different histological presentations, however the damaged tissue may provide the ideal microenvironment for invading treponemes thus disposing the animal to concurrent hoof conditions.19
Digital dermatitis has the same clinical presentation in beef cattle as it does in dairy cattle with similar predisposing factors, with the inclusion of potential introduction by contact with dairy animals (introduction of dairy-type steers at a feedlot, contact at livestock shows, etc). Whilst the prevalence of BDD in beef cattle does not approach the level seen in dairy cattle, it is being recognized more readily in recent years.20,21
In 1997, a severe virulent footrot was first described in sheep that failed to respond to formalin or zinc sulfate footbaths.3,22 Clinical presentation differed from ovine footrot as it was characterized by severe inflammatory lesions of the coronary bands which progressed to detachment of the hoof capsule. As with bovine digital dermatitis, it is evident that treponemes are a major bacterial component. Contagious Ovine Digital Dermatitis (CODD) is an emerging disease that has now become common in the UK. It is important to note that whilst detailed histological studies of lesions of CODD have not yet been completed, CODD is regarded as a distinct disease to that of ovine footrot or ovine interdigital dermatitis; this is evidenced by the failure of sheep with CODD to respond to treatments associated with footrot. Whilst treponemes were not originally identified in cases of ovine footrot and digital dermatitis, this should be reevaluated in light of improving culture and molecular techniques.23
The continued association of treponemes with lameness in hooved animals has also been documented during the emergence of abnormal hooves and lameness in a population of free-ranging Roosevelt elk in the US in 2008. Similar clinical presentation to CODD was observed including inflammatory lesions along the coronary band resulting in hoof sloughing, heel bulb ulceration and hyperplasia. Initial histological evaluations revealed alterations in the dermal structure similar to BDD.5 As with CODD and BDD, treponemes were identified within the lesions.4 No underlying systemic or bone related diseases were detected. Finally, treponemes were identified and cultured from the first reported cases of a “severe lameness problem” in a UK dairy goat herd.6 Collectively, these reports indicate that any cloven hoofed ruminant is susceptible to digital dermatitis, including domestic beef and dairy cattle, sheep, goats, and wild elk.4
Pathogens associated with digital dermatitis
Although no definitive etiological agent has yet been identified, it is clear that viral or fungal pathogens are not associated with DD.7,11,24 Further, DD is a polybacterial disease as evidenced by the detection of multiple different bacterial agents associated with clinical lesions, and their improved resolution in response to antibiotics. Whilst multiple bacterial agents have been routinely identified and cultured from active DD lesions, the most common bacteria associated with BDD and CODD are multiple phylotypes from the genus Treponema; phylotypes (PT) are defined as clusters of treponemes whose 16S rDNA sequence differs by ~2% from known species and are ≥99% similar to other members of their cluster.25 Finally, the evidence suggests that treponemes cultured from lesions associated with digital dermatitis have genetic similarities, whether they are identified in different countries, on different continents, or whether they are associated with bovine, ovine, cervine or caprine lesions.
The diversity and dynamics of treponemes and other bacterial agents associated with lesion progression in a closed bovine herd was recently characterized by high throughput DNA sequencing technologies.11 Of note, the bacterial microbiota of biopsies taken from lesions at different stages were statistically different, and as the lesion progressed, the abundance of Spirochaetaceae increased such that they accounted for 94.3% of sequences derived from a chronic lesion. All species of the family Spirochaetaceae were identified as belonging to the genus Treponema, containing 45 unique species of which 12 were predominant.11 These results confirm an array of previous studies which conclude that DD lesions are associated with multiple phylotypes of treponemes.2,26,27 Prevalence of the different phylotypes differs according to stage of lesion development as well as the location within the lesion. Multiple phylotypes of treponemes, identified by in situ hybridization, have been shown to be highly invasive with multiple different phylotypes detected in the same lesion.27 No individual phylotypes could be associated with a specific colonization pattern or type of lesion.28 Treponemes have also been detected in hair follicles and sebaceous glands, a possible route of entry.29
Despite their abundance, only a handful of treponemes have been successfully cultured to date. These include representatives of several clusters such as 1) T. medium/T. vincentii-like, 2) T. phagedenis-like, and 3) T. putidum/T. denticola-like which are grouped according to 16S rDNA and flaB2 gene homology. The first group is similar to T. vincentii, a pathogen associated with human periodontal disease whilst the second group of T. phagedenis-like treponemes is reported to be the one and same as the human genitalia commensal bacteria T. phagedenis.30 Human and bovine isolates of T. phagedenis from the US, the UK, and Sweden have >98% identical 16S rDNA sequence, similar enzyme activity profiles, growth tolerances, and physical appearance. The third group of T. putidum/T.denticola-like treponemes is now recognized as the unique species of T. pedis.31 All three groups have been cultured from lesions derived from cattle, sheep, elk and goats.22,32
Whilst no single phylotype dominates all lesions, T. phagedenis has been cultured from all stages of lesion development. It is hypothesized that different phylotypes dominate the lesion at different stages;27 treponemes dominating the early lesions most resembled uncultured, unidentified T. refringens-like PT1, PT2, PT3 (T. calligyrum-like) and a novel genomospecies closely related to T. refringens.11 In contrast, T. medium, T. pedis/PT8 and T. denticola were the most common treponeme operational taxonomic units identified in mature or chronic lesions. Multiple phylotypes (mean ranges from 7 to 15) are typically identified in each lesion.25,27,33
Whilst much has been made of the association of treponemes with DD lesions, it is theorized that a number of other bacteria are required to facilitate skin colonization, lesion development, and chronicity.2,27 Much of this evidence comes from the culture of these organisms along with treponemes from lesions, microscopic examination of the lesions (histologic evaluation, fluorescent in situ hybridization), and metagenomic sequencing. These findings are summarized in Table 1. Further evidence for their role in BDD includes the antibody response seen in cattle with active or recent BDD in which higher levels of reactive IgG to antigens from Porphyromonas, Fusobacterium, and Dichelobacter are detected (Wilson-Welder, unpublished data).34 The role of these bacteria as primary pathogens or secondary colonizers is not clear; the high prevalence of Dichelobacter in healthy feet indicates that D. nodusus alone is less likely to cause disease.2 However D. nodusus produces extracellular proteases assumed to be associated with tissue damage and can be readily codetected with treponemes in cows with interdigital dermatitis and heel horn erosion, and thus is hypothesized to act in synergy with treponemes to initiate bovine and ovine digital dermatitis.2,27,35,36 In support of this, a lower prevalence of Dichelobacter was identified in chronic lesions compared to acute lesions. This is not without precedent as D. nodusus and Fusobacterium necrophorum act synergistically to cause ovine footrot.
Treponemes associated with DD induce a humoral and cell mediated immune response. Serum antibody reacts with great affinity to whole-cell sonicates of treponemes isolated from lesions.37–41 Several researchers have attempted to use this as a predictor of animal exposure or digital dermatitis lesion status.42–44 The general conclusion is that serology is not suitable as a complete replacement for visual inspection of the hoof in bovine digital dermatitis, and has limited application to making herd level decisions. There is a wide range in response due to individual animal variability within groups demonstrating active lesions, recovered lesions, and among naïve groupings.39,44 The variable antibody response to treponemes also was observed in Washington elk (Wilson-Welder, unpublished data). Part of this variability may be explained by the different phylotypes of treponemes found in the DD lesions and the hypothesis that these populations shift over time11 and are spatially distributed within the lesion,45 and thus provide little or limited contact with the host immune system. Further theories as to the variability of the antibody response include potential immunological tolerance as treponemes are part of the normal intestinal flora,46,47 along with the variable nature of the DD treponemes themselves. Research demonstrated few cross-reacting epitopes amongst DD treponemes.40
Studies into the cell-mediated immune response elicited by BDD or CODD are limited. Studies using a bovine macrophage cell line incubated with a T. phagedenis isolated from BDD (Iowa strain 1A) showed increased expression of genes regulated by NFκB and other cell signaling associated molecules, increased expression of apoptosis associated molecules (BCL-2), downregulation of immune modulation pathways, antigen presentation, and cytoskeletal rearrangement, and wound healing pathways.48 This represents a single cell type interacting with a whole cell sonicate of a single bacterium present in the BDD lesion, giving just a small snapshot of the complexity of host–pathogen cross talk. In another study, analysis of total RNA transcripts in BDD lesions and normal skin with pathway analysis software indicated no activation or suppression of local immune response.49 Molecular signaling pathways increased in BDD lesions over normal skin include IL1β, a cytokine involved in early initiation of inflammation, and matrix metalloproteinase 13 which is secreted by many cell types and is key to tissue matrix remodeling. Interestingly, the expression of genes encoding keratin and keratin-associated proteins were downregulated in BDD.49 Cellular proliferation of peripheral blood mononuclear cells (PBMCs) occurred when they were incubated with treponemal antigen, a large percentage of which were γσ-Tcells.40 Whilst treponeme whole cell sonicate induced host inflammatory mediators in bovine foot skin fibroblasts, no significant changes were observed in bovine foot keratinocytes.50
Current understanding of disease transmission
The broad role of biosecurity has importance in prevention of transmission of infectious disease, including prevention of the spread of digital dermatitis.51 With herds without history of the disease, one should be aware of the status of herds from which replacements are sourced, whether the carrier employed has transported animals with digital dermatitis, and the handling of replacements upon arrival using quarantine housing. There is also a risk of transmission of DD from the comingling of sheep and cattle.35 The use of hoof trimmers is of benefit in preventing and controlling lameness in general, and improved conformation provides increased resistance to DD;52 however, a recent study has indicated the presence of treponeme DNA on trimming equipment, including isolation of a T. phagedenis-like isolate from a trimming knife after trimming an affected cow.53 Disinfection of the equipment resulted in decreased detection of DNA and negative culture, emphasizing the need for cleaning between animals and between farms to avoid inadvertent spread of the disease. As is frequently the case with biosecurity, the impact of available resources, personnel, time, and the perception of impact frequently affect implementation of control measures as well as the design and implementation of quarantine and elimination plans.35,52,54–57
The source of treponemes involved in DD has been examined with indications that environmental slurry and cow feces may serve as a source of exposure,58 with the oral cavity, colon, and rectum indicated as potential sites of colonization.46 However, of the >20 phylotypes of treponemes associated with BDD, none are considered part of the normal microbiota of the bovine gastrointestinal tract.58 Additionally, phylotypes associated with BDD were identified at such a low prevalence rate in feces and environmental slurry that their biological significance remains to be validated. Nevertheless, poor leg cleanliness is consistently associated with increased risk of DD.
Control measures should focus not only on limiting exposure to known risk factors, but also on curing existing DD lesions. The speed of detecting acute lesions and efficiency of treatment were key parameters in whether they became more severe or not. A fast transition from an acute lesion to a healing lesion is achieved by promoting early treatment, whilst a delayed transition from a healing lesion to an acute lesion is achieved by efficient footbath protocol.59 The persistence of treponemes in treated or resolving lesions may act as a continued source of infection; a foot initially observed with a chronic DD lesion which was considered “cured” was more likely to develop active DD than a foot initially free of DD. This may allow treponemes to persist at both the animal and herd level, despite the use of topical treatments, potentially contributing to the high recurrence rates (54%) observed. Topical treatments may only be useful in particular settings as spirochetes are not completely eradicated from the surface of lesions after treatment, such that relapses occur at 5–7 weeks when treated with a single topical application of oxytetracycline.13,15,60,61 Additional studies may shed further light on this possibility and could indicate the need to consider systemic treatments, as recognized for the treatment of CODD.36 In a study evaluating the timing of initial clinical disease, it was found that heifers experiencing DD prior to first calving were prone to develop recurrent lesions in subsequent lactations. This suggested a chronic infection status with the potential for transmission to susceptible herd mates, a possibility hinted at as well by the high level of recurrence rates (33%) observed.1,62
Numerous studies have been conducted to examine risk factors for DD; these include an association between disease expression and age at first calving, housing type, days in milk, parity, herd size, type of land cows access on a daily basis, flooring type where lactating cows walked, percent of cows born off the operation, use of a primary hoof trimmer, and lack of washing of trimming equipment between cows, and genetic contribution.64–66 Recent observations of the presence of DD Treponema spp. in association with other forms of lameness which were clinically characterized as nonhealing including toe necrosis, sole-ulcer, and white line disease, suggest the potential for colonization of physically compromised hoof tissues.67 These sites represent regions beyond those normally associated with DD lesions. The authors proposed a potential for these treponemes, on DD endemically affected farms, to play a role in the development of the nonhealing state.67 Similar organisms have been observed in multiple ulcerative lesion sites in different species (sheep,68 swine,69–71 horses,72–75 and cattle20), and their detection now in other non-infectious hoof diseases speaks to the opportunistic behavior demonstrated in affecting compromised tissues, and the ability of these treponemes to exacerbate different clinical issues.
Considerations to further understand the disease process of DD
Typical lesions associated with DD in bovines, ovines, and cervines are shown in Figures 1 and 2. Generally, bovine lesions are visually assessed, and 3–5 mm biopsies are taken for more detailed culture and histopathological studies, as in the area circled in Figure 1B, a lesion that was positive for the presence of spirochetes (Wilson-Welder, data not shown). However, further examination of the lesion highlighted in Figure 1B is provided in Figure 3A, which shows the extent of the lesion when visualized after dissecting between the bovine digits. Results illustrate a lesion that extends throughout the entire interdigital space and several millimeters below the skin surface.
BDD is a relatively novel disease that has rapidly emerged to be a leading cause of bovine lameness. Although significant progress has been made in identifying many of the clinical signs associated with BDD as well as lesion development, there is very little understanding on the initiation of the primary acute lesion and the complete resolution (if any) of a treated chronic lesion. Visual inspection is not sufficient as a means of detecting the early stages of lesion development, and the topical application of treatments to lesions such as that illustrated in Figure 3A is not sufficient to clear infection. The presence of layers of proliferative skin on DD lesions could have major implications for how infectious bovine lameness affects and stays endemic in modern herds.42 Our understanding of CODD, elk hoof disease and severe lameness in goats is even less and little, if any, data is available on lesion initiation and/or development.
Studies suggest that the persistence of treponemes in lesions of DD act as a source of recurrent infections, not only in the same animal but in naïve populations.52 Such persistence is a hallmark of disease associated with other treponemes including those involved with human periodontal disease and syphilis; indeed persistence is a hallmark of infection by many spirochetes. Thus, appropriate studies are required to supplement our current understanding and specifically address the causes of lesion initiation and the use of topical as well as systemic treatments to limit disease recurrence. These are not trivial tasks and ultimately require the use of appropriate animal models of infection.
BDD has been experimentally reproduced;76 DD is a polybacterial disease process, and no single etiological agent can reproduce the disease in experimentally infected bovines. Attempts to induce BDD lesions with cultures of T. phagedenis have been unsuccessful (Alt, unpublished data).77 However, when Holstein heifers of 14–16 months of age were treated such that their rear legs were subjected to prolonged moisture (maceration) and reduced access to air (closure), the inoculation of BDD lesion material resulted in the diagnosis of BDD histopathologically by 17 days postinfection in four of six legs.76 Use of a clonal isolate of a T. vincentii-like organism caused BDD in only one of four legs. These results highlight the need for the use of lesion material (and thus polybacterial material) as well as a suitably predisposed damaged foot surface to facilitate disease development. In modeling disease, while the native host is always best, near substitutes may be more practical. Mature bovines present considerable logistics for evaluation of hoofs on a daily basis. Other small ruminants (sheep or goats), having already demonstrated natural susceptibility to disease, may be a feasible alternative.
In contrast, another recent study failed to observe transmission from clinically affected cows cohoused with eight healthy heifers over a period of 8 weeks despite employing several housing and environmental modifications in an attempt to enhance transmission.33 These studies must be viewed in light of other data collected indicating the contribution of additional predisposing factors required for the development and expression of disease. Predisposing factors are complex and varied; they range from negative energy balance to poor hoof conformation.
DD is a polytreponemal disease. A relatively few number of DD treponemes are amenable to culture and thus available for a more comprehensive analysis at the molecular level. For most DD isolates, little work has been done on virulence attributes. It is clear that these treponemes comprise a group that have different physical appearance (in length, thickness, and number of flagella),10,16,26,78 different nutritional requirements (eg, use of fetal calf serum in medium compared to rabbit serum),79 host interactions,80,81 as well as the expression of different protein profiles.40,50 BDD treponeme isolates can be differentiated from bovine gastrointestinal treponeme isolates by the presence of tlyC,82 a hemolysin and putative virulence factor, but other virulence factors that contribute to pathogenesis are not known. These questions require the continued development of culture methods, genomic sequencing, and proteomics profiles of DD isolates. Such studies are confounded by the need to consider appropriate synergies with other nontreponemal bacterial isolates from DD lesions. Whilst similarities exist with the dental pathogens, drawing assumptions across isolates from such widely differing ecological niches may diminish unique attributes of the DD treponemes.
Concluding remarks
Globally recognized, lameness in animals is a major issue both from the standpoint of economic losses due to decreased production but also represents a serious animal welfare issue. The paradox of modern animal agriculture is that many of the husbandry practices intended to enhance performance also enhance predisposition for damaged hooves and infectious hoof disease transmission. Although the ideal situation is always complete elimination of a pathogen or disease, DD may need to be managed through the concept of endemic stability. In endemic stability, it is a balance of infection and disease, as individuals are exposed, immunity is developed, and for much of the population, disease is minimized.83 Individuals exhibiting disease are treated promptly and the environment is managed for low exposure levels. Since endemic stability requires that as the prevalence of infection increases the prevalence of disease decreases, pathogens that lend themselves to management through endemic stability tend to generate immune responses that are partial or that wane, resulting in repeated bouts of infection. Better knowledge about initiating events of DD and the role of the various bacterial pathogens in the infection would make management more precise. However, whilst this type of disease management is often the recourse for domestic livestock, it is detrimental to wildlife populations as there is little or no intervention for those individuals developing disease.
Acknowledgments
We thank Jim Fosse, Gearóid Sayers and Karen Mansfield for images of bovine, ovine, and cervine lesions respectively. We also thank the three anonymous reviewers for constructive critique. USDA is an equal opportunity provider and employer.
Disclosure
The authors report no conflicts of interest in this work.
References
Read DH, Walker RL. Papillomatous digital dermatitis (footwarts) in California dairy cattle: clinical and gross pathologic findings. J Vet Diagn Invest. 1998;10(1):67–76. | |
Knappe-Poindecker M, Gilhuus M, Jensen TK, Klitgaard K, Larssen RB, Fjeldaas T. Interdigital dermatitis, heel horn erosion, and digital dermatitis in 14 Norwegian dairy herds. J Dairy Sci. 2013;96(12):7617–7629. | |
Duncan JS, Angell JW, Carter SD, Evans NJ, Sullivan LE, Grove-White DH. Contagious ovine digital dermatitis: an emerging disease. Vet J. 2014; 201(3):265–268. | |
Clegg SR, Mansfield KG, Newbrook K, et al. Isolation of digital dermatitis treponemes from hoof lesions in wild North American elk (Cervus elaphus) in Washington State, USA. J Clin Microbiol. 2015;53(1):88–94. | |
Han S, Mansfield KG. Severe hoof disease in free-ranging Roosevelt elk (Cervus elaphus roosevelti) in southwestern Washington, USA. J Wildlife Dis. 2014;50(2):259–270. | |
Cheli R, Mortellaro C. Digital dermatitis in cattle. Proc 8th Int Meet Dis Cattle, Milan, Italy. 1974;8:208–213. | |
Rebhun WC, Payne RM, King JM, Wolfe M, Begg SN. Interdigital papillomatosis in dairy cattle. J Am Vet Med Assoc. 1980;177(5):437–440. | |
Blowey RW, Done SH, Cooley W. Observations on the pathogenesis of digital dermatitis in cattle. Vet Rec. 1994;135(5):115–117. | |
Losinger WC. Economic impacts of reduced milk production associated with papillomatous digital dermatitis in dairy cows in the USA. J Dairy Res. 2006;73(2):244–256. | |
Dopfer D, Koopmans A, Meijer FA, et al. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet Rec. 1997;140(24):620–623. | |
Krull AC, Shearer JK, Gorden PJ, Cooper VL, Phillips GJ, Plummer PJ. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect Immun. 2014;82(8):3359–3373. | |
Laven RA, Hunt H. Evaluation of copper sulphate, formalin and peracetic acid in footbaths for the treatment of digital dermatitis in cattle. Vet Rec. 2002;151(5):144–146. | |
Manske T, Hultgren J, Bergsten C. Topical treatment of digital dermatitis associated with severe heel-horn erosion in a Swedish dairy herd. Prevent Vet Med. 2002;53(3):215–231. | |
Holzhauer M, Bartels CJ, Dopfer D, van Schaik G. Clinical course of digital dermatitis lesions in an endemically infected herd without preventive herd strategies. Vet J. 2008;177(2):222–230. | |
Berry SL, Read DH, Famula TR, Mongini A, Dopfer D. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet J. 2012;193(3):654–658. | |
Walker RL, Read DH, Loretz KJ, Nordhausen RW. Spirochetes isolated from dairy cattle with papillomatous digital dermatitis and interdigital dermatitis. Vet Microbiol. 1995;47(3–4):343–355. | |
Nielsen BH, Thomsen PT, Green LE, Kaler J. A study of the dynamics of digital dermatitis in 742 lactating dairy cows. Prevent Vet Med. 2012;104(1–2):44–52. | |
Nielsen BH, Thomsen PT, Sorensen JT. A study of duration of digital dermatitis lesions after treatment in a Danish dairy herd. Acta Vet Scand. 2009;51:27. | |
Blowey RW, Done SH. Failure to demonstrate histological changes of digital or interdigital dermatitis in biopsies of slurry heel. Vet Rec. 1995;137(15):379–381. | |
Sullivan LE, Carter SD, Blowey R, Duncan JS, Grove-White D, Evans NJ. Digital dermatitis in beef cattle. Vet Rec. 2013;173(23):582. | |
Brown CC, Kilgo PD, Jacobsen KL. Prevalence of papillomatous digital dermatitis among culled adult cattle in the southeastern United States. Am J Vet Res. 2000;61(8):928–930. | |
Collighan RJ, Woodward MJ. Spirochaetes and other bacterial species associated with bovine digital dermatitis. FEMS Microbiol Lett. 1997; 156(1):37–41. | |
Davies IH, Naylor RD, Martin PK. Severe ovine foot disease. Vet Rec. 1999;145(22):646. | |
Bassett HF, Monaghan ML, Lenhan P, Doherty ML, Carter ME. Bovine digital dermatitis. Vet Rec. 1990;126(7):164–165. | |
Klitgaard K, Foix Breto A, Boye M, Jensen TK. Targeting the treponemal microbiome of digital dermatitis infections by high-resolution phylogenetic analyses and comparison with fluorescent in situ hybridization. J Clin Microbiol. 2013;51(7):2212–2219. | |
Choi BK, Nattermann H, Grund S, Haider W, Gobel UB. Spirochetes from digital dermatitis lesions in cattle are closely related to treponemes associated with human periodontitis. Int J Syst Bacteriol. 1997;47(1):175–181. | |
Rasmussen M, Capion N, Klitgaard K, et al. Bovine digital dermatitis: possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet Microbiol. 2012;160(1–2):151–161. | |
Cruz CE, Pescador CA, Nakajima Y, Driemeier D. Immunopathological investigations on bovine digital epidermitis. Vet Rec. 2005;157(26):834–840. | |
Evans NJ, Brown JM, Demirkan I, et al. Association of unique, isolated treponemes with bovine digital dermatitis lesions. J Clin Microbiol. 2009;47(3):689–696. | |
Wilson-Welder JH, Elliott MK, Zuerner RL, Bayles DO, Alt DP, Stanton TB. Biochemical and molecular characterization of Treponema phagedenis-like spirochetes isolated from a bovine digital dermatitis lesion. BMC Microbiol. 2013;13:280. | |
Evans NJ, Brown JM, Demirkan I, et al. Treponema pedis sp. nov., a spirochaete isolated from bovine digital dermatitis lesions. Int J Syst Evol Microbiol. 2009;59(Pt 5):987–991. | |
Collighan RJ, Naylor RD, Martin PK, Cooley BA, Buller N, Woodward MJ. A spirochete isolated from a case of severe virulent ovine foot disease is closely related to a Treponeme isolated from human periodontitis and bovine digital dermatitis. Vet Microbiol. 2000;74(3):249–257. | |
Capion N, Boye M, Ekstrom CT, Jensen TK. Infection dynamics of digital dermatitis in first-lactation Holstein cows in an infected herd. J Dairy Sci. 2012;95(11):6457–6464. | |
Moe KK, Yano T, Misumi K, et al. Detection of antibodies against Fusobacterium necrophorum and Porphyromonas levii-like species in dairy cattle with papillomatous digital dermatitis. Microbiol Immunol. 2010;54(6):338–346. | |
Knappe-Poindecker M, Gilhuus M, Jensen TK, Vatn S, Jorgensen HJ, Fjeldaas T. Cross-infection of virulent Dichelobacter nodosus between sheep and co-grazing cattle. Vet Microbiol. 2014;170(3–4):375–382. | |
Duncan JS, Grove-White D, Moks E, et al. Impact of footrot vaccination and antibiotic therapy on footrot and contagious ovine digital dermatitis. Vet Rec. 2012;170(18):462. | |
Demirkan I, Walker RL, Murray RD, Blowey RW, Carter SD. Serological evidence of spirochaetal infections associated with digital dermatitis in dairy cattle. Vet J. 1999;157(1):69–77. | |
Elliott MK, Alt DP. Bovine immune response to papillomatous digital dermatitis (PDD)-associated spirochetes is skewed in isolate reactivity and subclass elicitation. Vet Immunol Immunopathol. 2009;130(3–4):256–261. | |
Moe KK, Yano T, Misumi K, et al. Analysis of the IgG immune response to Treponema phagedenis-like spirochetes in individual dairy cattle with papillomatous digital dermatitis. Clin Vaccine Immunol. 2010;17(3):376–383. | |
Trott DJ, Moeller MR, Zuerner RL, et al. Characterization of Treponema phagedenis-like spirochetes isolated from papillomatous digital dermatitis lesions in dairy cattle. J Clin Microbiol. 2003;41(6):2522–2529. | |
Walker RL, Read DH, Loretz KJ, Hird DW, Berry SL. Humoral response of dairy cattle to spirochetes isolated from papillomatous digital dermatitis lesions. Am J Vet Res. 1997;58(7):744–748. | |
Gomez A, Anklam KS, Cook NB, et al. Immune response against Treponema spp. and ELISA detection of digital dermatitis. J Dairy Sci. 2014;97(8):4864–4875. | |
Murray RD, Downham DY, Demirkan I, Carter SD. Some relationships between spirochaete infections and digital dermatitis in four UK dairy herds. Res Vet Sci. 2002;73(3):223–230. | |
Vink WD, Jones G, Johnson WO, et al. Diagnostic assessment without cut-offs: application of serology for the modelling of bovine digital dermatitis infection. Prevent Vet Med. 2009;92(3):235–248. | |
Moter A, Leist G, Rudolph R, et al. Fluorescence in situ hybridization shows spatial distribution of as yet uncultured treponemes in biopsies from digital dermatitis lesions. Microbiology. 1998;144(Pt 9):2459–2467. | |
Evans NJ, Timofte D, Isherwood DR, et al. Host and environmental reservoirs of infection for bovine digital dermatitis treponemes. Vet Microbiol. 2012;156(1–2):102–109. | |
Shibahara T, Ohya T, Ishii R, et al. Concurrent spirochaetal infections of the feet and colon of cattle in Japan. Aust Vete J. 2002;80(8):497–502. | |
Zuerner RL, Heidari M, Elliott MK, Alt DP, Neill JD. Papillomatous digital dermatitis spirochetes suppress the bovine macrophage innate immune response. Vet Microbiol. 2007;125(3–4):256–264. | |
Scholey R, Evans N, Blowey R, et al. Identifying host pathogenic pathways in bovine digital dermatitis by RNA-Seq analysis. Vet J. 2013; 197(3):699–706. | |
Evans NJ, Brown JM, Scholey R, et al. Differential inflammatory responses of bovine foot skin fibroblasts and keratinocytes to digital dermatitis treponemes. Vet Immunol Immunopathol. 2014;161(1–2):12–20. | |
Mee JF, Geraghty T, O’Neill R, More SJ. Bioexclusion of diseases from dairy and beef farms: risks of introducing infectious agents and risk reduction strategies. Vet J. 2012;194(2):143–150. | |
Relun A, Lehebel A, Bruggink M, Bareille N, Guatteo R. Estimation of the relative impact of treatment and herd management practices on prevention of digital dermatitis in French dairy herds. Prevent Vet Med. 2013;110(3–4):558–562. | |
Sullivan LE, Blowey RW, Carter SD, et al. Presence of digital dermatitis treponemes on cattle and sheep hoof trimming equipment. Vet Rec. 2014;175(8):201. | |
Whay H, Barker Z, Leach K, Main D. Promoting farmer engagement and activity in the control of dairy cattle lameness. Vet J. 2012;193(3):617–621. | |
Ward W. Why is lameness in dairy cows so intractable? Vet J. 2009; 180(2):139–140. | |
Main D, Leach K, Barker Z, et al. Evaluating an intervention to reduce lameness in dairy cattle. J Dairy Sci. 2012;95(6):2946–2954. | |
Bell N, Bell M, Knowles T, Whay H, Main D, Webster A. The development, implementation and testing of a lameness control programme based on HACCP principles and designed for heifers on dairy farms. Vet J. 2009;180(2):178–188. | |
Klitgaard K, Nielsen MW, Ingerslev HC, Boye M, Jensen TK. Discovery of bovine digital dermatitis-associated Treponema spp. in the dairy herd environment by a targeted deep-sequencing approach. Appl Environ Microbiol. 2014;80(14):4427–4432. | |
Dopfer D, Holzhauer M, Boven MV. The dynamics of digital dermatitis in populations of dairy cattle: Model-based estimates of transition rates and implications for control. Vet J. 2012;193(3):648–653. | |
Berry SL, Read DH, Walker RL, Famula TR. Clinical, histologic, and bacteriologic findings in dairy cows with digital dermatitis (footwarts) one month after topical treatment with lincomycin hydrochloride or oxytetracycline hydrochloride. J Am Vet Med Assoc. 2010;237(5):555–560. | |
Mumba T, Dopfer D, Kruitwagen C, Dreher M, Gaastra W, van der Zeijst BA. Detection of spirochetes by polymerase chain reaction and its relation to the course of digital dermatitis after local antibiotic treatment in dairy cattle. Zentralbl Veterinarmed B. 1999;46(2):117–126. | |
van Amstel SR, van Vuuren S, Tutt CL. Digital dermatitis: report of an outbreak. J S Afr Vet Assoc. 1995;66(3):177–181. | |
Holzhauer M, Hardenberg C, Bartels CJ, Frankena K. Herd- and cow-level prevalence of digital dermatitis in the Netherlands and associated risk factors. J Dairy Sci. 2006;89(2):580–588. | |
Somers JG, Frankena K, Noordhuizen-Stassen EN, Metz JH. Risk factors for digital dermatitis in dairy cows kept in cubicle houses in The Netherlands. Prevent Vet Med. 2005;71(1–2):11–21. | |
Scholey R, Ollier W, Blowey R, Murray R, Carter S. Determining host genetic susceptibility or resistance to bovine digital dermatitis in cattle. Adv Animal Biosci. 2010;1(01):2. | |
Onyiro OM, Andrews LJ, Brotherstone S. Genetic parameters for digital dermatitis and correlations with locomotion, production, fertility traits, and longevity in Holstein-Friesian dairy cows. J Dairy Sci. 2008;91(10):4037–4046. | |
Evans NJ, Blowey RW, Timofte D, et al. Association between bovine digital dermatitis treponemes and a range of ‘non-healing’ bovine hoof disorders. Vet Rec. 2011;168(8):214. | |
Moore LJ, Woodward MJ, Grogono-Thomas R. The occurrence of treponemes in contagious ovine digital dermatitis and the characterisation of associated Dichelobacter nodosus. Vet Microbiol. 2005;111(3–4):199–209. | |
Karlsson F, Klitgaard K, Jensen TK. Identification of Treponema pedis as the predominant Treponema species in porcine skin ulcers by fluorescence in situ hybridization and high-throughput sequencing. Vet Microbiol. 2014;171(1):122–131. | |
Svartström O, Karlsson F, Fellström C, Pringle M. Characterization of Treponema spp. isolates from pigs with ear necrosis and shoulder ulcers. Vet Microbiol. 2013;166(3):617–623. | |
Pringle M, Fellström C. Treponema pedis isolated from a sow shoulder ulcer. Vet Microbiol. 2010;142(3):461–463. | |
Moe KK, Yano T, Kuwano A, Sasaki S, Misawa N. Detection of treponemes in canker lesions of horses by 16S rRNA clonal sequencing analysis. J Vet Med Sci. 2010;72(2):235–239. | |
Nagamine CM, Castro F, Buchanan B, Schumacher J, Craig LE. Proliferative pododermatitis (canker) with intralesional spirochetes in three horses. J Vet Diagn Invest. 2005;17(3):269–271. | |
Rashmir-Raven AM, Black SS, Rickard LG, Akin M. Papillomatous pastern dermatitis with spirochetes and Pelodera strongyloides in a Tennessee Walking Horse. J Vet Diagn Invest. 2000;12(3):287–291. | |
Sykora S, Brandt S. Occurrence of Treponema DNA in equine hoof canker and normal hoof tissue. Equine Vet J. Epub August 13, 2014. | |
Gomez A, Cook NB, Bernardoni ND, et al. An experimental infection model to induce digital dermatitis infection in cattle. J Dairy Sci. 2012; 95(4):1821–1830. | |
Pringle M, Bergsten C, Fernstrom LL, Hook H, Johansson KE. Isolation and characterization of Treponema phagedenis-like spirochetes from digital dermatitis lesions in Swedish dairy cattle. Acta Vet Scand. 2008;50:40. | |
Dopfer D, Anklam K, Mikheil D, Ladell P. Growth curves and morphology of three Treponema subtypes isolated from digital dermatitis in cattle. Vet J. 2012;193(3):685–693. | |
Evans NJ, Brown JM, Demirkan I, et al. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet Microbiol. 2008;130(1–2):141–150. | |
Edwards AM, Dymock D, Woodward MJ, Jenkinson HF. Genetic relatedness and phenotypic characteristics of Treponema associated with human periodontal tissues and ruminant foot disease. Microbiology. 2003;149(Pt 5):1083–1093. | |
Edwards AM, Dymock D, Jenkinson HF. From tooth to hoof: treponemes in tissue-destructive diseases. J Appl Microbiol. 2003;94(5):767–780. | |
Evans NJ, Brown JM, Murray RD, et al. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl Environ Microbiol. 2011; 77(1):138–147. | |
Green LE, George T. Assessment of current knowledge of footrot in sheep with particular reference to Dichelobacter nodosus and implications for elimination or control strategies for sheep in Great Britain. Vet J. 2008;175(2):173–180. | |
Santos TM, Pereira RV, Caixeta LS, Guard CL, Bicalho RC. Microbial diversity in bovine papillomatous digital dermatitis in Holstein dairy cows from upstate New York. FEMS Microbiol Ecol. 2012;79(2):518–529. | |
Sayers G, Marques PX, Evans NJ, et al. Identification of spirochetes associated with contagious ovine digital dermatitis. J Clin Microbiol. 2009;47(4):1199–1201. | |
Koniarova I, Orsag A, Ledecký V. [The role anaerobes in dermatitis digitalis et interdigitalis in cattle]. Vet Med. 1992;38(10):589–596. Slovak. | |
Schlafer S, Nordhoff M, Wyss C, et al. Involvement of Guggenheimella bovis in digital dermatitis lesions of dairy cows. Vet Microbiol. 2008;128(1–2):118–125. | |
Wyss C, Moter A, Choi BK, et al. Treponema putidum sp. nov., a medium-sized proteolytic spirochaete isolated from lesions of human periodontitis and acute necrotizing ulcerative gingivitis. Int J Syst Evol Microbiol. 2004;54(Pt 4):1117–1122. | |
Schroeder CM, Parlor KW, Marsh TL, Ames NK, Goeman AK, Walker RD. Characterization of the predominant anaerobic bacterium recovered from digital dermatitis lesions in three Michigan dairy cows. Anaerobe. 2003;9(3):151–155. | |
Ohya T, Yamaguchi H, Nii Y, Ito H. Isolation of Campylobacter sputorum from lesions of papillomatous digital dermatitis in dairy cattle. Vet Rec. 1999;145(11):316–318. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.