Back to Journals » Clinical Ophthalmology » Volume 8
The diurnal and nocturnal effects of travoprost in normal-tension glaucoma
Authors Seibold LK, Kahook M
Received 23 August 2014
Accepted for publication 22 September 2014
Published 31 October 2014 Volume 2014:8 Pages 2189—2193
DOI https://doi.org/10.2147/OPTH.S73125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Leonard K Seibold, Malik Y Kahook
Department of Ophthalmology, University of Colorado Eye Center, Aurora, CO, USA
Purpose: To determine the diurnal and nocturnal effects of travoprost with sofZia® (Travatan Z® [TZ]) on intraocular pressure (IOP) and ocular perfusion pressure (OPP) in patients with normal-tension glaucoma (NTG).
Methods: Twenty-seven subjects with NTG were admitted to an inpatient sleep laboratory for three 24-hour sessions monitoring IOP, blood pressure (BP), and heart rate every 2 hours in the habitual position (diurnal period: upright; nocturnal period: supine). Baseline IOP and OPP levels were compared to those during active treatment with TZ and 3 days after stopping the medication. OPP was calculated as 2/3 [diastolic BP + 1/3 (systolic BP – diastolic BP)] – IOP.
Results: TZ significantly reduced the mean diurnal and nocturnal IOP levels compared to baseline at all time points. During treatment, mean IOP decreased from 17.1±3.4 to 14.7±3.0 mmHg during the diurnal period (P<0.01) and from 19.9±3.6 to 18.8±3.5 mmHg during the nocturnal period (P<0.01). Once treatment was discontinued, mean IOP remained at levels significantly less than baseline during both the diurnal (15.6±3.2 mmHg) and nocturnal (18.7±3.7 mmHg) periods. Mean OPP was not significantly changed with treatment during either period.
Conclusion: In this population of NTG patients, TZ significantly lowers IOP at all time points throughout the diurnal and nocturnal periods. The treatment effect on IOP endures for up to 3 days after the last dose. Treatment did not significantly improve OPP.
Keywords: ocular perfusion pressure, intraocular pressure, 24-hour, diurnal, nocturnal, sofZia®
Introduction
The pathogenesis of normal-tension glaucoma (NTG) remains poorly understood and is likely multifactorial, including vascular dysregulation and structural abnormalities.1,2 The Collaborative Normal-Tension Glaucoma Study demonstrated that, similar to other forms of open-angle glaucoma (OAG), intraocular pressure (IOP) does indeed have a significant role in the pathogenesis of NTG.3 Patients with NTG achieving a 30% IOP reduction were less likely to develop progression of visual field defects or glaucomatous optic nerve head changes. Treatment of NTG has therefore hinged on the same principle of IOP reduction by any means available, with topical medications being a readily available and commonly utilized choice.
The IOP-lowering efficacy and safety of prostaglandin analogues (PGAs) in ocular hypertension (OHTN) and OAG has been well documented.4 A recent meta-analysis of seven clinical trials by Dubiner and Noecker found that the PGA travoprost (Travatan®; Alcon Laboratories, Inc., Fort Worth, TX, USA) lowered IOP by, on average, 30% in these populations.5 We have previously reported significant IOP reductions during treatment with travoprost with sofZia® (Travatan Z® [TZ]; Alcon Laboratories, Inc.) throughout a 24-hour period in patients with OHTN or OAG.6 The efficacy of travoprost in an NTG population has been less robust, with IOP reductions ranging from 16.1%–25.1% based on a relative paucity of data.7–9 The IOP-lowering effects of travoprost throughout the 24-hour cycle in NTG patients is poorly characterized, and monitoring these effects in the habitual position has not been reported previously.
Even less understood are the effects of travoprost and other PGA medications on ocular perfusion pressure (OPP). A growing body of literature supports the clinical relevance of OPP in glaucoma.10 This metric is of particular interest for NTG, in which compromised perfusion of the optic nerve head may play a role in the pathogenesis of the disease.1 Recently, the Low-pressure Glaucoma Treatment Study (LoGTS) found that, although brimonidine and timolol both effectively lowered IOP to a similar degree in NTG patients, subjects receiving timolol were more likely to develop visual field progression.11 While OPP was not specifically reported, blood pressure (BP) was significantly lower in the timolol-treated group, suggesting a potential mechanism of IOP-independent glaucoma progression. In newly diagnosed OAG, Quaranta et al found that both brimonidine and timolol significantly lowered BP, with brimonidine causing a significant decrease in mean 24-hour OPP.12
In the current study, we aimed to evaluate the 24-hour effects of TZ on IOP in NTG patients and assess the endurance of effect after three missed doses. Furthermore, we sought to investigate the medication’s effect on OPP throughout a 24-hour period.
Methods
Prior to the start of the study, approval was obtained from the Colorado Multiple Institutional Review Board, and the tenets of the Declaration of Helsinki were adhered to. All subjects provided informed consent prior to study enrollment. A total of 27 consecutive patients with NTG were recruited during routine examinations at the University of Colorado Eye Center, Aurora, CO, USA. Inclusion criteria included both male and female patients of any ethnicity with an existing or new diagnosis of NTG. NTG was defined by the presence of characteristic glaucomatous optic nerve head changes and/or visual field defects with all recorded IOP measurements ≤ 22 mmHg. Exclusion criteria included angle closure glaucoma, anatomically narrow angle on gonioscopy, a history of untreated IOP >22 mmHg, pregnant females or those planning to become pregnant, current smokers, abnormal sleep habits, inability to safely endure medication washout period, current use of medical marijuana, and history of cystoid macular edema, uveitis, or herpes simplex viral infection.
All subjects being treated with topical glaucoma medications at the time of recruitment were asked to discontinue their medications for a minimum of 4 weeks, irrespective of drug class. Subjects not on glaucoma therapy at the time of recruitment were permitted to begin with the first of three 24-hour study visits. Each study visit was conducted in a private inpatient room at the University of Colorado Hospital Clinical and Translational Research Center, where patients remained for a full 24-hour period. The first visit served as baseline and was conducted after washout of medications, if applicable. After the first session was completed, each subject was started on a once-nightly regimen of TZ to be given in each eye at 9 pm. A follow-up phone call was made 2 weeks after the initiation of therapy to ensure tolerance and adherence with the medication. The second study visit was performed after completing 4 weeks of treatment with TZ. In order to determine persistence of effect, the medication was then stopped, and subjects returned for a third study visit after three missed doses.
Subjects were encouraged to conduct normal daytime activities during each inpatient study visit. During the nocturnal period of 10 pm to 6 am, subjects were asked to remain lying in bed except for bathroom privileges. An ad libitum diet was provided during the daytime hours. At each 2-hour interval, BP and heart rate (HR) were recorded simultaneously, followed by IOP measurement. For each measurement, the subject was required to be resting in the appropriate habitual position for at least 10 minutes. For the diurnal period, all measurements (7 am, 9 am, 11 am, 1 pm, 3 pm, 5 pm, 7 pm, and 9 pm) were performed in the sitting, upright position, while those taken during the nocturnal period (11 pm, 1 am, 3 am, and 5 am) were done with the subject supine. All readings were performed by a trained ophthalmic technician or registered nurse. After topical proparacaine 0.5% administration, a calibrated Model 30™ pneumatonometer (Reichert Inc., Depew, NY, USA) was utilized for IOP measurements. For any standard deviation >1.0, the reading was not recorded and the measurement was repeated. Measurements were taken on both eyes, providing each eye qualified for the study. BP and HR were measured using a standard automated sphygmomanometer. OPP was calculated according to previously described equations.6 The means of IOP and OPP were compared for each 2-hour time interval as well as for the entire diurnal and nocturnal period. Mixed models were utilized with various contrasts reported through linear combinations of the models’ parameters. These models were exclusively designed to take correlation from repeated measurements into account. Statistical analyses were completed using SAS 9.3 software (SAS Institute Inc., Cary, NC, USA). The criterion for statistical significance was P<0.05.
Results
Of the 27 subjects enrolled, 19 were female and eight were male, with a mean age of 65.7 years (range: 45–84 years). Based on self-reported ethnicity, subjects were composed of 14 Caucasians, ten African Americans, two Asians, and one Hispanic. Only one patient was not taking glaucoma medications at the time of enrollment; the remainder underwent the required washout period of medications. Each subject completed the entirety of the study. There were no drug-related serious adverse events, and all participants reported good medication tolerance.
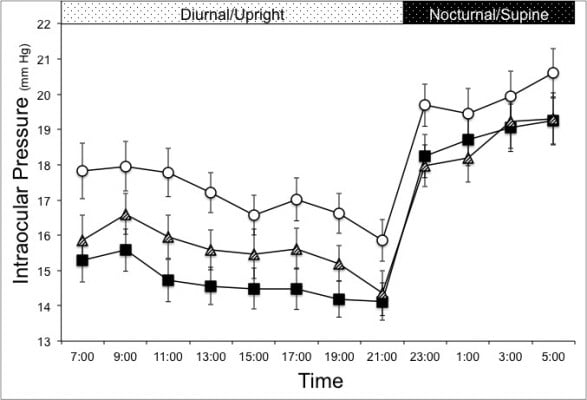
During treatment with TZ, IOP was significantly reduced from 17.1±3.4 to 14.7±3.0 mmHg during the diurnal period and from 19.9±3.6 to 18.8±3.5 mmHg during the nocturnal period. Once the medication was discontinued for 3 days, mean IOP remained significantly reduced from baseline during both periods and statistically similar to levels during active treatment (P=0.2 diurnal period, P=0.45 nocturnal period). The mean IOP during the diurnal and nocturnal periods are displayed in Table 1. Figure 1 displays the IOP profiles from each 24-hour visit. Mean IOP during TZ therapy was significantly reduced at each time point during the diurnal period (upright) and, to a lesser degree, during the nocturnal period (supine). After three missed doses, the IOP levels at all time points during the diurnal period increased a minor amount, yet remained significantly less than baseline. Over the nocturnal period, there was no significant change in IOP reduction up to 84 hours after the last dose taken.
The mean OPPs during each study session over the diurnal and nocturnal periods are listed in Table 2. During the diurnal period, OPP increased a small but statistically insignificant amount, from 77.4±10.4 mmHg at baseline to 79.0±10.0 mmHg during treatment. No effect on OPP was noted with treatment during the nocturnal period. Figure 2 contains the OPP profiles for each 24-hour study visit. During the diurnal period, OPP trended above baseline levels at each time point; however, only the 5 pm time point reached statistical significance. Diastolic and systolic BP were unchanged during treatment compared to baseline levels. No significant change in HR was detected during any study visit.
Discussion
This study presents the 24-hour effects of the PGA TZ on IOP and OPP in patients with NTG. For every 2-hour interval measured throughout a 24-hour cycle, IOP was significantly decreased from baseline during TZ therapy. The magnitude of IOP reduction was greatest during the diurnal period (upright position) where the degree of IOP fluctuation was also minimized during treatment. Furthermore, our findings demonstrate a sustained response of IOP reduction at all time points after three missed doses. While our findings are not altogether unexpected, they confirm and expand on the current knowledge of the 24-hour clinical effects of TZ in NTG.
Limited studies have been performed to date regarding the efficacy of TZ in patients with NTG. In 2009, Suh et al reported an 18.8%–25.1% IOP reduction with travoprost in 45 patients with unilateral NTG after 12 months of treatment.8 In the longest-term follow-up, Inoue et al recently reported a 16% IOP reduction with travoprost in NTG patients at 3 years.7 In a randomized controlled trial, Ang et al analyzed the effect of travoprost on diurnal IOP (8 am to 5 pm) in 54 NTG patients.9 At 6 months, the average diurnal IOP was significantly reduced by 16.1%, with only 32.9% achieving an IOP reduction >20%. Nomura et al performed the only study reporting on the 24-hour effects of travoprost in NTG.13 In their study, mean 24-hour IOP was reduced from 12.9 mmHg to 10.3 mmHg (20% reduction). Of note in their protocol, subjects were awoken and walked to a slit lamp to perform IOP readings in an upright position overnight.
Our findings were also consistent with an IOP reduction far short of the 30% seen with travoprost in patients with OHTN or “high-pressure” OAG.5 In fact, our mean IOP reduction was only 14% during the diurnal period and 11% for the entire 24-hour period. We can propose a few causes for the small disparities of our results compared to prior work. First, our study demographics were unique, with a largely non-Asian population composed primarily of Caucasians (52%) and African Americans (37%). Second, our protocol was unique in obtaining measurements with patients in the habitual position for each time period. We propose that, because of this, our findings may be more in line with actual IOP levels outside a clinical setting. The pneumatonometry method of IOP measurement was employed throughout the trial to allow supine IOP readings over the nocturnal period, whereas all previous studies utilized Goldmann applanation tonometry. Finally, we utilized a 4-week washout of medications; however, some evidence suggests that the IOP-lowering effects of PGAs may persist up to 6 weeks.14
The exact duration of PGA medication’s effect on IOP is unclear, but it has been shown to exceed the 24-hour dosing schedule. In a Phase II trial of OAG and OHTN patients, Traverso et al found that IOP remained significantly lower than baseline, although less pronounced, up to 48 hours after the last dose taken of latanoprost and tafluprost.15 After 2 weeks of travoprost therapy with and without benzalkonium chloride (BAK), Gross et al measured IOP in OAG and OHTN patients every 12 hours after the last dose.16 Mean IOP remained significantly decreased from baseline for up to 60 hours after the last dose. Sit et al found travoprost with BAK significantly lowered IOP at all time points of a 24-hour session after two missed doses of medication.17 Similarly, we have demonstrated a sustained effect of TZ after three missed doses in OAG and OHTN patients.6 From the present study, our results demonstrate that the IOP-lowering effect of TZ also persisted in an NTG population up to 84 hours after the last dose. This may be clinically relevant in patients who occasionally miss one or more doses of medication.
Our analysis failed to show a significant increase in OPP during treatment with TZ. Despite this, the trend was for a small increase in OPP during the diurnal period only, with statistical significance being reached at only a single time interval. This is in contrast to a previous study of OAG and OHTN patients, in which TZ achieved a significant increase in OPP during the diurnal period, but not the nocturnal period.6 The 24-hour effect of travoprost on OPP has not been previously reported in an NTG population. For comparison, limited work has been done to assess the effect of other PGAs on OPP, with mixed results. One study, by Quaranta et al found a small but significant increase in circadian OPP with latanoprost treatment, while bimatoprost treatment failed to show a change.18 In a crossover NTG study by Liu et al latanoprost achieved a significant increase in OPP, whereas brimonidine did not.19 In contrast, a later study of latanoprost showed no effect on BP or circadian OPP in NTG patients.20
A few limitations of the current study must be noted. First, due to the lack of a separate or placebo control group, the subjects served as their own control. Similarly, the noncomparative design makes it difficult to make true comparisons between the effects of travoprost with BAK and TZ. Thus, our data may only be compared with previously published data using unique populations and protocols. Next, it should be noted that true OPP requires measurement of the BP within the ophthalmic artery. Due to the difficult and invasive nature of this measurement, we used brachial BP to calculate an estimate of the true OPP, which is a method frequently used in the literature. Furthermore, static BP readings were measured as opposed to the use of dynamic, ambulatory BP monitoring.
Conclusion
TZ achieved significant IOP reduction throughout the diurnal and nocturnal period in this population of patients with NTG. The effect persisted after three missed doses with no significant attenuation during both the diurnal and nocturnal periods. In this normotensive population, OPP was statistically unchanged over the diurnal and nocturnal period.
Acknowledgments
The authors have received research support for this study from Alcon Laboratories, Inc. (Fort Worth, TX, USA), and National Institutes of Health/National Center for Advancing Translational Sciences, Colorado Clinical and Translational Sciences Institute Grant Number UL1 TR000154. The contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Disclosure
Malik Y Kahook is a consultant for Alcon Laboratories, Inc. Both authors received research support for this study from Alcon Laboratories, Inc. The authors report no other conflicts of interest in this work.
References
Mozaffarieh M, Flammer J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharmacol. 2013;13(1):43–49. | ||
Burgoyne CF, Downs JC. Premise and prediction-how optic nerve head biomechanics underlies the susceptibility and clinical behavior of the aged optic nerve head. J Glaucoma. 2008;17(4):318–328. | ||
[No authors listed]. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126(4):487–497. | ||
Bean GW, Camras CB. Commercially available prostaglandin analogs for the reduction of intraocular pressure: similarities and differences. Surv Ophthalmol. 2008;53 Suppl1:S69–S84. | ||
Dubiner HB, Noecker R. Sustained intraocular pressure reduction throughout the day with travoprost ophthalmic solution 0.004%. Clin Ophthalmol. 2012;6:525–531. | ||
Seibold LK, Kahook MY. The diurnal and nocturnal effect of travoprost with sofZia on intraocular pressure and ocular perfusion pressure. Am J Ophthalmol. 2014;157(1):44–49.e1. | ||
Inoue K, Iwasa M, Wakakura M, Tomita G. Effects of BAK-free travoprost treatment for 3 years in patients with normal tension glaucoma. Clin Ophthalmol. 2012;6:1315–1319. | ||
Suh MH, Park KH, Kim DM. Effect of travoprost on intraocular pressure during 12 months of treatment for normal-tension glaucoma. Jpn J Ophthalmol. 2009;53(1):18–23. | ||
Ang GS, Kersey JP, Shepstone L, Broadway DC. The effect of travoprost on daytime intraocular pressure in normal tension glaucoma: a randomised controlled trial. Br J Ophthalmol. 2008;92(8):1129–1133. | ||
Quaranta L, Katsanos A, Russo A, Riva I. 24-hour intraocular pressure and ocular perfusion pressure in glaucoma. Surv Ophthalmol. 2013;58(1):26–41. | ||
Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S; Low-Pressure Glaucoma Study Group. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011; 151(4):671–681. | ||
Quaranta L, Gandolfo F, Turano R, et al. Effects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucoma. Invest Ophthalmol Vis Sci. 2006;47(7):2917–2923. | ||
Nomura Y, Nakakura S, Moriwaki M, Takahashi Y, Shiraki K. Effect of travoprost on 24-hour intraocular pressure in normal tension glaucoma. Clin Ophthalmol. 2010;4:643–647. | ||
Stewart WC, Holmes KT, Johnson MA. Washout periods for brimonidine 0.2% and latanoprost 0.005%. Am J Ophthalmol. 2001;131(6): 798–799. | ||
Traverso CE, Ropo A, Papadia M, Uusitalo H. A phase II study on the duration and stability of the intraocular pressure-lowering effect and tolerability of Tafluprost compared with latanoprost. J Ocul Pharmacol Ther. 2010;26:97–104. | ||
Gross RL, Peace JH, Smith SE, et al. Duration of IOP reduction with travoprost BAK-free solution. J Glaucoma. 2008;17:217–222. | ||
Sit AJ, Weinreb RN, Crowston JG, Kripke DF, Liu JH. Sustained effect of travoprost on diurnal and nocturnal intraocular pressure. Am J Ophthalmol. 2006;141:1131–1133. | ||
Quaranta L, Pizzolante T, Riva I, Haidich AB, Konstas AG, Stewart WC. Twenty-four-hour intraocular pressure and blood pressure levels with bimatoprost versus latanoprost in patients with normal-tension glaucoma. Br J Ophthalmol. 2008;92:1227–1231. | ||
Liu CJ, Ko YC, Cheng CY, et al. Changes in intraocular pressure and ocular perfusion pressure after latanoprost 0.005% or brimonidine tartrate 0.2% in normal-tension glaucoma patients. Ophthalmology. 2002;109:2241–2247. | ||
Ishibashi S, Hirose N, Tawara A, Kubota T. Effect of latanoprost on the diurnal variations in the intraocular and ocular perfusion pressure in normal tension glaucoma. J Glaucoma. 2006;15:354–357. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





