Back to Journals » International Journal of General Medicine » Volume 7
Study of urinary leukotriene E4 levels in children with acute asthma
Authors Abd El-Motaleb GS, Amer A, Elawa G, Abd Elfattah M
Received 27 October 2013
Accepted for publication 11 December 2013
Published 3 March 2014 Volume 2014:7 Pages 131—135
DOI https://doi.org/10.2147/IJGM.S56660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Ghada Saad Abd El-Motaleb,1 Alraohaa Ahmed Ahmed Abou Amer,1 Gamal Mohamed Elawa,2 Mohamed Abo Alsood Abd Elfattah3
1Department of Pediatrics, 2Department of Clinical Pathology, Faculty of Medicine, Benha University, Benha, Qalyubia, Egypt; 3Department of Pediatrics, Ministry of Health Hospital, Cairo, Egypt
Objective: The goal of this study was to evaluate the impact of urinary leukotriene E4 (ULTE4) in asthmatic children during acute asthma exacerbation. Also, we wanted to correlate it with the total serum (TS) immunoglobulin (Ig) E level in relation to asthma severity.
Methods: This prospective study was conducted in the emergency room of the Department of Pediatrics, Benha University Hospital in Benha, Egypt, from November 2011 to May 2012. In this study, 63 children with acute asthma exacerbation (group I) and 25 healthy children as the control (group II) were included. Their ages ranged between 3–14 years. All children were subjected to a full medical history, and an emphasis was placed on the symptoms of asthma exacerbation and a complete clinical examination. TS IgE and ULTE4 were measured by an enzyme-linked immunosorbent assay after receiving the parents' consent. The LTE4 concentration was measured in urine samples obtained at the onset of admission and adjusted by urinary creatinine concentrations.
Results: The ULTE4 levels were significantly higher in cases compared to the controls (309.7±97.1 versus 14.5±5.7 pg/mg creatinine, respectively). It correlated positively to both the TS IgE and asthma severity in the cases. Also, the TS IgE levels increased significantly in cases (392.1±309.7 IU/mL), when compared to the controls (45.5±22.1 IU/mL).
Conclusion: The ULTE4 and the TS IgE levels are significantly elevated during acute asthma episodes in children. The significant positive correlation between the severity of these attacks and the ULTE4 levels makes it a good noninvasive marker for monitoring acute asthma exacerbations and follow-up of the inflammatory process in asthmatic children.
Keywords: Egypt, ULTE4 in children, acute asthma
Introduction
In asthma, many cells (such as mast cells, eosinophils, and T lymphocytes) and cellular elements (such as cysteinyl leukotrienes [LTs], cytokines, and histamines) play a role in airway hyperresponsiveness.1
LTs play a central pathophysiological role in asthma, particularly in specific subgroups of patients with asthma.2 High cysteinyl LT concentrations have been detected in different body fluids, such as sputum, urine, and exhaled breath condensate from patients with asthma.3
The diagnosis and management of asthma in young children are currently based on subjective clinical features and medical examinations. Therefore, there is a lot of interest in noninvasive techniques to assess inflammation – especially in children – as inflammatory markers in exhaled breath condensate (such as nitric oxide and leukotriene E4 [LTE4]) and blood (such as eosinophils and total and specific IgE).4
The aim of this study was to evaluate the impact of urinary (U) LTE4 in asthmatic children during acute asthma exacerbation. Also, our aim was to correlate it with the total serum (TS) IgE level in relation to asthma severity.
Methods
This prospective cross-sectional study was conducted in the emergency room of Department of Pediatrics, Benha University Hospital, between November 2011 and May 2012. Eighty-eight children were included in this work. They were divided into two groups. In group I, there were 63 asthmatic children during the acute attack (first 24 hours). Their ages ranged from 3–14 years, with a mean of 7.9±3.5 years. In group II, 25 healthy children were matched for age and sex as a control group. Their mean age was 6.7±3.3 years. Patients with other chronic chest, cardiovascular, or neurological disease were excluded from this study.
Ethics permission was granted by the Ethics Committee of the Faculty of Medicine at Benha University, in September 2011. An informed, written consent was also taken from all the children’s parents before the beginning of the study.
Both groups were subjected to the following: 1) full medical history with an emphasis on the symptoms of asthma exacerbation, such as breathing difficulties, coughing, and a detailed drug-use history, (for example, bronchodilators and parenteral corticosteroids); 2) complete clinical examination; 3) measurement of LTE4 in urine (5 mL of midstream urine was collected in plastic tubes and stored in −20°C. Urine samples were taken from each patient at the onset of admission to the emergency room and before using any medication; and 4) measurement of the TS IgE (1 mL blood sample obtained under aseptic technique, centrifuged immediately [Clay Adams® Dynac® II Centrifuge], and with serum, stored at −20°C until further use).
Principle of assay of LTE4 by enzyme-linked immunosorbent assay (ELISA)
This assay (ACE EIA kit, Cayman Chemical, Ann Arbor, MI, USA) was based on the competition between the LTE4 and the LTE4–acetylcholinesterase (AChE) conjugate (LTE4 tracer) for a limited amount of LTE4 antiserum. Because the concentration of the LTE4 tracer was held constant while the concentration of the LTE4 varied, the amount of LTE4 tracer that was able to bind the LTE4 antiserum was inversely proportional to the concentration of the LTE4 in the well. This antibody-LTE4 complex was bonded to a mouse monoclonal antirabbit IgG that had been previously attached to the well. The plate was washed to remove any unbound reagents and then Ellman’s reagent (which contains the substrate to AChE) (Cayman Chemical) was added to the well. The product of this enzymatic reaction had a distinct yellow color and absorbed strongly at 420 nm. The intensity of this color determined spectrophotometrically was proportional to the amount of LTE4 tracer bound to the well, which was inversely proportional to the amount of free LTE4 present in the well during the incubation, according to the equation by Haus et al:5
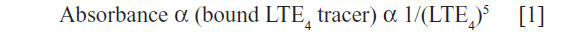
Measurement of TS IgE by ELISA
The total IgE was measured by using the Quantitative ELISA Kit (Pishtaz Teb Zaman Diagnostics, Tehran, Iran) according to the manufacturer’s instructions. The absorbance was read on a microplate reader, reading at the 450 nm wavelength. The detection limit of the IgE by this kit is 1 IU/mL; the intra- and interassay coefficients of variation for quality control samples were 6% and 7.35%, respectively.6
The SPSS statistical software, version 10.0 (IBM, Armonk, NY, USA) was used for data analysis. The mean and the standard deviation are descriptive values for quantitative variables. The Student’s t-test and the nonparametric t-test (the Mann–Whitney U-test) were used for comparing the means of the two independent groups. The analysis of variance compared means of more than two independent groups. The Pearson correlation analysis and Spearman’s rho correlation measured the association between the quantitative variables.
Results
The mean age of cases in this study was 7.9±3.5 years; in the control group, it was 6.7±3.3 years. In group I, 43 were males (68.3%) and 20 were females (31.7%); the ratio was 2.1:1. In group II, 18 were males (72.0%) and seven were females (28.0%) (Table 1).
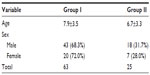  | Table 1 General characteristics of the studied groups |
The distribution of cases, according to asthma severity, was as follows: mild (55.6%); moderate (31.7%); and severe (12.7%). Viral upper respiratory tract infection was the most common trigger causing asthma exacerbation in 44% of cases. A positive family history of asthma and atopy was found in 46% of cases. The history of atopy was around 85%. In these cases, 61% had allergic rhinitis, 17.4% had allergic dermatitis, and 6.6% had a history of food and drug allergy.
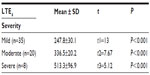
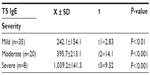
We found that there was a highly significant difference between the levels of ULTE4 between cases (309.7±97.1 pg/mg creatinine) and the control (14.5±5.7 pg/mg creatinine) group. The ULTE4 levels had a positive correlation with asthma severity (Table 2). The TS IgE levels among group I members were significantly elevated to 392.1±309.7 IU/mL with a very wide range (57–1,215 IU/mL) than in group II’s 45.5±22.1 IU/mL and positively correlated with asthma severity (Table 3).
There was a highly significant positive correlation between the ULTE4 levels and the TS IgE levels among group I (Figure 1). There was no significant impact of age or sex on TS IgE and ULTE4 levels among asthmatic cases.
  | Figure 1 Correlation between urinary LTE4 and TS IgE among group I. |
Discussion
The predominance of male cases (43:20 with a ratio of 2.1:1) in our study agreed with de Marco et al (2000),7 when a large study – conducted in 16 countries with more than 18,659 asthma patients – showed that asthma was more prevalent in males than females throughout childhood. The Mandhane et al (2005)8 study confirmed these results, finding that males more often developed childhood wheeze than females.
The present study demonstrated that the distribution of cases according to bronchial asthma severity was as follows: mild (55.6%); moderate (31.7%); and severe cases (12.7%). Mild was the most prevalent. This distribution of asthma severity in Egypt is consistent with the pattern reported in several countries, as concluded by Chipps et al (2006)9 and Simões et al (2010).10 Also, Hossny et al (2009)11 confirmed these results in a study of 422 asthmatic children living in Cairo and its suburbs. Their results were as follows: 88.3% had mild asthma; 10.2%, moderate; and 1.5%, severe.
The ULTE4 in the present study showed a highly significant increase (309.7±97.1 pg/mg creatinine) in group I when compared to group II (14.5±5.7 pg/mg creatinine). Also, the ULTE4 levels showed a highly significant positive correlation with asthma severity in group I: mild versus moderate, P<0.001; mild versus severe, P<0.001; and moderate versus severe, P<0.001.
In the Kawagishi et al (2001)12 study on asthmatic patients without attack, the values of ULTE4 were significantly higher in the asthmatic patients (113.6±9.7 pg/mg creatinine) than in the healthy control (67.8±4.7 pg/mg creatinine), and the level of ULTE4 was positively correlated with the severity of the disease. Yoshikawa et al (2004)13 confirmed this where ULTE4 levels during an acute attack (median 476 pg/mg creatinine) and during the stable condition (median 332 pg/mg creatinine) were significantly higher than those of the controls (median 233 pg/mg creatinine).
This was confirmed also by He et al (2009)14 who found that the ULTE4 levels of asthmatic children at acute and convalescence phases were significantly higher than the control group (P<0.01) and were significantly reduced at the convalescence phase when compared to the acute phase (P<0.01).
Our results showed that the TS IgE levels were increased more significantly in cases (392.1±309.7 IU/mL) than in the controls (45.5±22.1 IU/mL) (t=8.83 and P<0.001). Also, they were increased significantly with asthma severity. There was a significant positive correlation between TS IgE and ULTE4 (P<0.001) in group I, but both TS IgE and ULTE4 levels had an insignificant relation to age and sex among cases.
These results are confirmed by Haselkorn et al (2010).15 While evaluating the relationships between patient characters, for example, age, sex, and the atopic indicators of asthma in children, Haselkorn reported allergic rhinitis in approximately two-thirds of patients. This finding was compatible with our cases (62%); up to 25% of the Haselkorn patients had atopic dermatitis (17% in our study). Severe or difficult-to-treat asthma in children is associated with high IgE levels. All cases with severe asthma had the highest level of TS IgE in our study.
In the present study, the history of atopy was positive in 85% of cases. We detected that all cases with a low TS IgE (seven out of the 63 cases included in the study) had a history of atopy, while the remaining cases with the high TS IgE level had a history of atopy in only 47 cases (83% of the cases). So, from these results, we suggested that the elevation of TS IgE level is a good indicator of atopy (good sensitivity), and the decrease of TS IgE level even to low levels can’t exclude atopy (poor specificity).
In agreement with our results, Azzazy et al (2008)16 demonstrated the overlapping in TS IgE levels in cases with asthma and controls with an insignificant difference in the mean TS IgE level between atopic and nonatopic asthma (313.3±154.4 and 283.2±119.8 IU/L).
In the Fajraoui et al (2008)17 study, they proved our results by showing that the TS IgE in that both allergic and control groups were overlapped. The measure of the TS IgE level is not helpful for a diagnosis of allergic (respiratory) diseases, because it has a quite good sensibility but a poor specificity and poor negative predictive value. So, the test was more sensible and less specific in children. The essential role of IgE in asthma was found by Gergen et al (2009),18 regardless the atopic status.
Conclusion
The ULTE4 and the TS IgE levels are significantly elevated during acute asthma episodes in children. The significant positive correlation between the severity of these attacks and the ULTE4 levels make it a good, simple noninvasive marker for monitoring the severity of acute asthma, and it can be used in a follow-up of the inflammatory process in asthmatic children. Due to the high cost of this test, however, its use is still limited.
Disclosure
The authors report no conflicts of interest in this work.
References
Lipkowitz MA, Navarra T. History of allergy and immunology. In: The Encyclopedia of Allergies. 2nd ed. New York: Facts on File; 2001:167. | |
Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. | |
Montuschi P, Mondino C, Koch P, Barnes PJ, Ciabattoni G. Effect of a leukotriene receptor antagonist on exhaled leukotriene E4 and prostanoids in children with asthma. J Allergy Clin Immunol. 2006;118(2):347–353. | |
Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11(7):577–584. | |
Haus M, Heese HD, Weinberg EG, Potter PC, Hall JM, Malherbe D. The influence of ethnicity, an atopic family history, and maternal ascariasis on cord blood serum IgE concentrations. J Allergy Clin Immunol. 1988;82(2):179–189. | |
Westcott JY, Maxey KM, MacDonald J, Wenzel SE. Immunoaffinity resin for purification of urinary leukotriene E4. Prostaglandins Other Lipid Mediat. 1998;55(5–6):301–321. | |
de Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med. 2000;162(1):68–74. | |
Mandhane PJ, Greene JM, Cowan JO, Taylor DR, Sears MR. Sex differences in factors associated with childhood- and adolescent-onset wheeze. Am J Respir Crit Care Med. 2005;172(1):45–54. | |
Chipps BE, Spahn JD, Sorkness CA, et al. Variability in asthma severity in pediatric subjects with asthma previously receiving short-acting beta2-agonists. J Pediatr. 2006;148(4):517–521. | |
Simões SM, Cunha SS, Barreto ML, Cruz AA. Distribution of severity of asthma in childhood. J Pediatr (Rio J). 2010;86(5):417–423. Portuguese [with English abstract]. | |
Hossny EM, Hasan ZE, Allam MF, Mahmoud ES. Analysis of the filed data of a sample of Egyptian children with bronchial asthma. Egyptian Journal of Pediatric Allergy Immunology. 2009;7(2):59–64. | |
Kawagishi Y, Oosaki R, Mita H, et al. [Clinical significance of measurement of urinary leukotriene E4 in asthmatic patients without attack]. Arerugi. 2001;50(11):1096–1101. Japanese [with English abstract]. | |
Yoshikawa K, Matsui E, Inoue R, et al. Urinary leukotriene E4 and 11-dehydro-thromboxane B2 excretion in children with bronchial asthma. Allergology International. 2004;53(2):127–134. | |
He MJ, Chen Q, Liu JM. [Urinary leukotrience E(4) level in children with asthma]. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11(11):909–912. Chinese [with English abstract]. | |
Haselkorn T, Szefler SJ, Simons FE, et al; TENOR Study Group. Allergy, total serum immunoglobulin E, and airflow in children and adolescents in TENOR. Pediatr Allergy Immunol. 2010;21(8):1157–1165. | |
Azzazy EA, Bahgat M, Soliman M, Kadry H. Urinary leukotriene level in patients with bronchial asthma. Egyptian Journal of Medical Microbiology. 2008;17(1):161–168. | |
Fajraoui N, Charfi MR, Khouani H, Abouda M, Kerkenil Y, Zouari B. [Contribution of serum total immunoglobulin E measurement in the diagnosis of respiratory allergic diseases]. Tunis Med. 2008; 86(1):32–37. French [with English abstract]. | |
Gergen PJ, Arbes SJ Jr, Calatroni A, Mitchell HE, Zeldin DC. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2009;124(3):447–453. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.