Back to Journals » Local and Regional Anesthesia » Volume 8
Simulation in teaching regional anesthesia: current perspectives
Authors Udani A, Kim TE, Howard S, Mariano E
Received 18 April 2015
Accepted for publication 25 May 2015
Published 11 August 2015 Volume 2015:8 Pages 33—43
DOI https://doi.org/10.2147/LRA.S68223
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Stefan Wirz
Ankeet D Udani,1 T Edward Kim,2,3 Steven K Howard,2,3 Edward R Mariano2,3
On behalf of the ADAPT (Anesthesiology-Directed Advanced Procedural Training) Research Group
1Department of Anesthesiology, Duke University School of Medicine, Durham, NC, USA; 2Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Stanford, CA, USA; 3Anesthesiology and Perioperative Care Service, Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA
Abstract: The emerging subspecialty of regional anesthesiology and acute pain medicine represents an opportunity to evaluate critically the current methods of teaching regional anesthesia techniques and the practice of acute pain medicine. To date, there have been a wide variety of simulation applications in this field, and efficacy has largely been assumed. However, a thorough review of the literature reveals that effective teaching strategies, including simulation, in regional anesthesiology and acute pain medicine are not established completely yet. Future research should be directed toward comparative-effectiveness of simulation versus other accepted teaching methods, exploring the combination of procedural training with realistic clinical scenarios, and the application of simulation-based teaching curricula to a wider range of learner, from the student to the practicing physician.
Keywords: regional anesthesia, simulation, medical education, ultrasound, nerve block, simulator
Introduction
The list of teaching and learning methods in modern medical education is exhaustive. The age-old model of observation followed by attempt (ie, “see one, do one, teach one”) still exists and is still considered common in residency and fellowship training.1 Other modalities available to today’s medical learner include formal didactic lectures and multimedia learning (eg, mobile devices, textbooks, and Internet).2–4 Traditionally, teaching methods have been introduced with minimal studies of efficacy, if any. Today, new methods are often held to a higher standard and are required to demonstrate superiority over some existing comparator. Those methods with proven efficacy may be integrated into medical education curricula, either individually or in combination with other methods as appropriate.
Simulation can be defined as something that is made to look, feel, or behave like something else especially when applied to research or education.5 The use of simulation to mimic real life in the educational setting has arguably many origins but is closely tied to Kolb’s experiential learning theory.6 The educational experience provided by simulation is often hands-on, practical, provides immediate feedback, and allows for repetition. Training with simulation does no harm to patients; errors can be allowed to occur, can even be scheduled, and can provide realistic experiences managing common and rare situations which differs to “training by chance” where exposure is limited to real life cases that may or may not occur for every trainee. In the medical education setting, simulation-based interventions are now mature with a wide variety of applications.7 For procedural skills, deliberate practice in a simulated-setting is one example of an effective teaching strategy that has been used for specific skills training in central venous catheter insertion, subarachnoid block placement, and laryngoscopy.8 Medical residents trained to place central venous catheters in simulation improve their clinical performance in the intensive care unit.9 The translation of knowledge, skills, and attitudes from the simulation-based classroom to clinical care is important to show effectiveness or efficacy and drive curricular change.10
Regional anesthesia inherently requires precise procedural performance due to target nerve locations near vital structures (eg, blood vessels, pleura, organs, and nerves themselves) and seems naturally suited for the incorporation of simulation within the training curricula. Further, the recent evolution of ultrasound guidance in the practice of regional anesthesia has created great demand for training in this imaging modality for physicians who completed training more than a decade ago and are still in clinical practice.11 The American Society of Regional Anesthesia and Pain Medicine (ASRA) and European Society of Regional Anesthesia and Pain Therapy (ESRA) have published joint committee guidelines for training in ultrasound-guided regional anesthesia, and the ASRA–ESRA guidelines suggest that simulation play an important role.12 Although there is widespread use of the modality, the evidence basis for simulation in regional anesthesia training is not completely established.2 In this review, we provide an up-to-date summary of the literature related to the use of simulation in regional anesthesia education and assess its effectiveness.
Literature review
A search of the MEDLINE database (PubMed.gov; United States National Library of Medicine, National Institutes of Health, Bethesda, MD, USA) using “regional anesthesia”, “simulation”, “regional anesthesia simulator”, “regional anesthesiology simulation”, “regional anesthesiology simulator”, “nerve block simulator”, and “nerve block simulation”, was conducted between January and March 2015, and resulted in 393 citations. Three of the authors (TK, AU, and EM) excluded non-English language, veterinary, nerve conduction (without nerve blockade), and magnetic resonance imaging articles and eliminated any duplicate citations based on the search terms and phrases; 64 articles remained. The reference lists of pertinent articles were also manually searched and revealed 15 additional articles not included in the original MEDLINE search. The final sample of 79 articles was critically reviewed for simulation-based educational interventions and their effectiveness.
Simulation-based educational interventions
Fourteen articles published to date study the effect on learners who underwent a simulation-based educational intervention in regional anesthesia (Table 1).13–26 Out of the 14, only studies by Niazi et al,15 Baranauskas et al,20 and Udani et al14 tested a simulation-based teaching strategy against an equivalent control group not receiving simulation; all three studies enrolled anesthesiology trainees.14,15,20 Based on the results of Niazi et al, residents in anesthesiology who received 1 hour of simulation training on needling and proper hand–eye coordination using ultrasound were more successful than a control group receiving no simulation, as assessed by blocks performed on real patients.15 Baranauskas et al studied different durations of simulation training and the potential effect on learners, including 0 hours of simulation.20 Students with 2 hours of simulation training in needling with ultrasound performed faster and with fewer technical flaws than students with 1 hour of simulation training. Additionally, those with 1 hour of training performed better than those with 0 hours of simulation training. Udani et al presented a randomized study in which residents are assigned to receive simulation-based deliberate practice teaching or a base curriculum without simulation to learn subarachnoid blocks.14 In this study, performance scores using a task checklist improved in the participants receiving simulation-based teaching. However, there may not be a translational benefit as there is no difference between groups in the time required for participants to place subarachnoid blocks in actual patients.
  | Table 1 Simulation-based educational interventions |
Table 1 includes other studies that on some level assess effectiveness of simulation-based educational interventions, but the ability to discern the impact of simulation alone in these studies is limited by methodology. Woodworth et al, Gasko et al, Friedman et al, and Liu et al describe controlled studies.13,17,19,22 However, the interventions under study are more complex than just incremental simulation-based teaching compared to a control group without simulation. For example, Woodworth et al include a teaching video in addition to simulation training, which is then compared to a control group.13 Gasko et al compare a combination of CD-ROM teaching material with simulation versus CD-ROM teaching alone versus simulation teaching alone.17 Friedman et al compare a high-fidelity simulator versus a low-fidelity epidural simulator without a comparison to no simulator.19 Although these studies demonstrate the benefits of enhanced and more rigorous training, the results cannot be attributed entirely to the introduction of simulation. Liu et al evaluate three different types of simulators for regional anesthesia and conclude that novice practitioners decrease the number of errors in a simulated block with each additional practice attempt in simulation, regardless of the type of simulator used.22
Moore et al, Garcia-Tomas et al, Ouanes et al, and Bretholz et al describe comprehensive regional anesthesia curricula and demonstrate their effectiveness.16,18,21,25 These interventions all include simulation-based teaching but are combined with other teaching strategies (eg, web-based tutorials, journal clubs, anatomy labs, etc); thus it is not possible to identify the specific contribution of simulation. Furthermore, studies lack a control group. Kim et al, Cheung et al, and Brenner et al use a single simulation-based teaching intervention, not in the context of a comprehensive curriculum, but do not include a control group comparison.23,24,26 Due to the nature of all participants’ receiving some type of teaching, the reported effectiveness is mostly positive. Participants report feeling more comfortable with proceedures, and post-intervention written test scores increase. The studies by Garcia-Tomas et al and Ouanes et al show post-intervention objective structured clinical examination (OSCE) scores also increase.18,21 Brenner et al report that interprofessional debriefings in their crisis management course lead to richer discussions.26 However, Moore et al show that although written test scores improve after implementation of their educational curriculum, there is no difference in block accuracy or efficiency as assessed in a simulator.16 Bretholz et al also report that the initial increase in comfort after their educational intervention is not sustained one month later.25
Novel simulator design
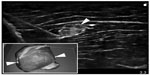
To date, 18 articles describe the design of a novel regional anesthesia simulator (Table 2).27–44 Simulators used for part-task training (eg, phantoms) in ultrasound-guided regional anesthesia vary based on the materials used and indications (ie, tasks to be taught). Inorganic materials are common in commercially available phantoms but often lack realistic tactile sensation and haptic feedback and do not allow for injection of liquid solutions as is common in regional anesthesia techniques (Figures 1 and 2). These phantoms are useful for teaching procedural steps, dexterity, target identification, and needle guidance. In contrast, organic phantoms (ie, meat) arguably produce the most realistic sonoanatomy and tactile sensation and do allow for injection and even catheter insertion, but they are not reusable and must be replaced for subsequent training sessions (Figure 3).
  | Table 2 Novel simulator design |
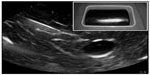  | Figure 1 Sample sonogram of a nonanatomic inorganic phantom for ultrasound-guided regional anesthesia. |
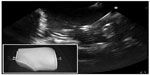  | Figure 2 Sample sonogram of an anatomic inorganic phantom for ultrasound-guided regional anesthesia. |
Since it is not possible to present every model used for regional anesthesia practice, for the purpose of this article we define a novel simulator design as a new, previously undescribed product employing innovative technology to represent the realistic scenario of performing a regional anesthesia procedure. The novel simulator design takes one of the following forms: 1) a physical model (ie, phantom); 2) virtual reality model; 3) robot-assisted model; or 4) environmental modification. Only Niazi et al,32 Lim et al,34 Lee et al,28 and Morse et al27 report an effect on learners attributable to the use of their novel simulators. The other 14 articles are solely descriptions of simulator design. None of the studies that report an effect on learners include a control group against which simulation-based teaching is compared. Rather, these studies focus on before and after test comparisons and show positive results associated with training on the novel simulators described. Lim et al demonstrate that after using their virtual reality simulator, participants’ skills in identifying surface landmarks for block placement improve.34 Lee et al demonstrate that participants’ time performing an epidural block decreases with 20 repetitions on their simulator.28 Morse et al use a crossover study design to show that performance is more consistent and that learning is quicker using a robot-assisted regional anesthesia technique in simulation when compared to a traditional, manual technique using practitioner’s hands.27
Use of a simulated environment as an experimental setting
Eleven articles present a new medical device or evaluate an established nerve block technique in a simulated environment (Table 3).45–55 Examples include primarily case reports on devices or techniques such as Luer connectors, echogenic needles, needle guides, “air test” for inferring perineural catheter tip location, and a hand-on-syringe technique.47–50,52,53 Only Kilicaslan et al,49 Whittaker et al,45 Neal et al,46 Johnson et al,47 and Gupta et al48 provide effectiveness data. Simulation itself is not evaluated for efficacy, and studies that include a control group expose both the control and intervention groups to the simulated environment. Reported outcomes include improvements in knowledge, skills, and/or behaviors with the use of the new device or technique. Neal et al46 evaluate the use of a treatment checklist for the management of local anesthetic systemic toxicity (LAST). The authors describe creating a simulated, clinical environment utilizing a mannequin to represent a clinical patient who receives an inadvertent toxic dose of intravascular local anesthetic. The learners, anesthesiology residents, and fellows, are inserted into the simulated crisis with or without a LAST treatment checklist. In their findings, the authors demonstrate that physician trainees using a treatment checklist make better medical decisions related to the critical management of LAST than trainees who do not use a checklist.
  | Table 3 Use of a simulated environment as an experimental setting |
Other published topics related to simulation and regional anesthesia
The remaining articles span a broad range of topics, and none test the effectiveness or efficacy of simulation-based teaching in regional anesthesia. Some authors collect the needs of training regional anesthesiologists and establish metrics to assess performance, using simulators.56–60 Others use a simulated environment to calculate procedural learning curves and observe practitioner ergonomics.61–67 Although these articles touch on the topic of medical education in regional anesthesia, we consider them outside the scope of the present review on simulation-based educational interventions and their effectiveness.
Discussion
Although simulation-based medical education has been shown to be effective for specific applications, our review reveals that similar evidence in regional anesthesia training is limited. We especially note a lack of comparative evidence studying the effectiveness of a simulation-based teaching strategy versus a proper control: participants’ receiving identical teaching as the intervention group less simulation-based instruction. This would mean that control group participants should have an equal amount of time in an educational setting even when they are not receiving simulation-based instruction. Medical education research is expected to show efficacy or comparative-effectiveness against an established method, or else educators may presume “if you teach [students], they will learn”.68 We know that the addition of simulation-based instruction automatically means extra training, but we cannot assume that this extra training automatically leads to the acquisition of new knowledge or skills.
We encourage the development, implementation, and scientific investigation of comprehensive regional anesthesia training curricula. From the work published to date, we are unable to deduce the incremental effectiveness (or ineffectiveness) of simulation-based regional anesthesia education, although we acknowledge that it has face validity. The description of novel simulators and development of new regional anesthesia techniques in a simulated environment represent just the first step to fully assess the role of simulation in teaching the knowledge, skills, and behaviors necessary for regional anesthesia competency.
Recently, regional anesthesia has been evolving further into the medical subspecialty of regional anesthesiology and acute pain medicine (RAAPM), and guidelines have been established for fellowship training.69 However, teaching strategies are not clearly recommended or identified. Learners are taught using various techniques, most commonly as observers who transition to active participants in the apprenticeship model. A mix of didactic, multimedia, and simulation-based learning would augment this apprenticeship. The effectiveness of the variations in the RAAPM curricula is largely unknown, and curricula may very well be institution-specific for many reasons (eg, faculty and resources available).70,71
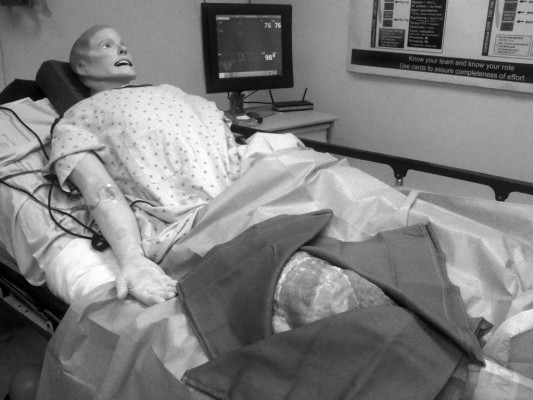
We see many advantages to simulation-based education in RAAPM. The key elements of simulation-based education, namely repetitive practice, targeted feedback, self-reflection, and avoiding harm to patients make it a useful teaching strategy. The simulated environment is well-suited for providing learners with the time and means to gain effective feedback and reflect on their performance; something that is difficult to obtain when taking care of real patients as time in the clinical setting is often limited and rushed. Many aspects of RAAPM are procedure based, and practice in simulation can recreate a realistic experience. We believe RAAPM may be the ideal specialty to embrace “hybrid” simulation (Figure 4), a combination of part-task training and mannequin-based simulation to facilitate procedural practice in the context of life-like scenarios (eg, the anxious patient, a vasovagal response, or even LAST). Repetitive practice in a controlled, simulated environment has been shown to lead to better procedural performance in the clinical setting.9 This is one of the tenets of deliberate practice, an effective use of simulation-based medical education.14 As educators, we hope that a student’s mistakes will be made in the simulated environment, and corrected prior to actual patient care. Another advantage of simulation-based education is that it exemplifies principles of adult learning and Kolb’s experiential learning theory. In our review of published articles, it is clear that learners of all stages in medical school, residency, fellowship, and clinical practice react and enjoy participating in simulation-based education even though it may initially cause some anxiety.72,73
Alternatively, there may be disadvantages to simulation-based education in RAAPM. First, one may assume that a large capital investment in equipment and time is required.74 In our review, we find that this may not be true as many novel low-cost simulator designs have been described to help keep costs to a minimum while maintaining fidelity and achieving learning objectives.30,33,36,75 Time in simulation is time away from clinical care. However, training guidelines established by the Accreditation Council for Graduate Medical Education for anesthesiology residents now require time in simulation annually, and simulation is included in the requirements for maintenance of board certification in anesthesiology. There is momentum to encourage novice physicians to practice in simulation prior to engaging in clinical care. The same may be said for physicians who have already completed their training and are trying to learn a new technique such as ultrasound-guided peripheral nerve blockade. Simulation may provide a more effective alternative to other methods currently available (eg, continuing medical education or industry-sponsored workshops), and self-teaching is still widespread in this population.76 There may be costs involved for practicing physicians to participate in continuing education as well as loss of income. In our opinion, the time spent in simulation may be a solid investment since a complication prevented by practice in simulation may actually save time and decrease complications in clinical practice.
There are several limitations to our review. The search terms related to regional anesthesiology, regional anesthesia, simulation, and simulation-based education have many pseudonyms that may have resulted in an incomplete literature search and inability to evaluate every study exploring the effectiveness of simulation-based education in regional anesthesia. Each of the authors completed his own web-based and manual searches to generate as comprehensive a reference list as was feasible. We define simulation broadly in this review and have included published articles that describe any type of simulated environment or training, including as little as 5 minutes in simulated-training,13 for the sake of completeness. In reality, we believe effective simulation-based education in RAAPM necessitates stricter criteria including personal feedback, repetitive practice, and student reflection. These are unique characteristics of simulation that have the potential to augment learning.
We as educators see additional benefits of simulation-based education. As anesthesiologists who specialize in RAAPM, the scope is not limited to just performance of nerve blocks. While achieving mastery in a new technical skill is a measurable and achievable result with simulation, this should not ever be a physician’s final goal. Studies by Neal et al46 investigating the use of emergency checklists during a simulated LAST crisis and Brenner et al26 employing similar crisis management simulations for pain physicians demonstrate the potential role of simulation in teaching professional practice far beyond mere technical skills. In RAAPM, the frontier for simulation-based education should evolve to include teaching difficult patient interactions (eg, demented elderly with hip fracture), ethical dilemmas (eg, wrong side blocks), interdisciplinary team-based care, safety culture, and more.
As technological advances in regional anesthesia and analgesia emerge, such as new robot-assisted procedures, medications, equipment, and techniques, they will require rigorous testing followed by an effective means to update training. The simulated environment is ideal for trialing these innovations, comparing them to current practices, and proving them effective prior to full implementation in clinical care. When there is sufficient evidence to support a change in clinical practice, simulation may even be able to facilitate dissemination and implementation.
In summary, the emerging subspecialty of RAAPM represents an opportunity to critically evaluate the current methods of teaching regional anesthesia techniques and the practice of acute pain medicine. To date, there have been a wide variety of simulation applications in this field, and efficacy has largely been assumed. However, a thorough review of the literature reveals that effective teaching strategies, including simulation, are not yet established completely, in RAAPM. Future research should be directed toward comparative-effectiveness of simulation versus other accepted teaching methods, exploring the combination of procedural training with realistic clinical scenarios, and the application of simulation-based teaching curricula to a wider range of learner from the student to the practicing physician.
Disclosure
Dr Mariano has received unrestricted educational program funding paid to his institution from Halyard Health (formerly I-Flow/Kimberly-Clark; Lake Forest, CA, USA) and B Braun (Bethlehem, PA, USA). These companies had no input into any aspect of the present study design and implementation; data collection, analysis and interpretation; or manuscript preparation. The authors report no conflicts of interest in this work.
References
Rodriguez-Paz JM, Kennedy M, Salas E, et al. Beyond “see one, do one, teach one”: toward a different training paradigm. Qual Saf Health Care. 2009;18(1):63–68. | |
Nix CM, Margarido CB, Awad IT, et al. A scoping review of the evidence for teaching ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2013;38(6):471–480. | |
Chelly JE, Greger J, Gebhard R, Hagberg CA, Al-Samsam T, Khan A. Training of residents in peripheral nerve blocks during anesthesiology residency. J Clin Anesth. 2002;14(8):584–588. | |
Broking K, Waurick R. How to teach regional anesthesia. Curr Opin Anaesthesiol. 2006;19(5):526–530. | |
Merriam-Webster Collegiate Dictionary. 11th ed. Springfield (MA): Merriam-Webster, Inc.; 2003. | |
Fanning RM, Gaba DM. The role of debriefing in simulation-based learning. Simul Healthc. 2007;2(2):115–125. | |
Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10–28. | |
McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706–711. | |
Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403. | |
McGaghie WC, Draycott TJ, Dunn WF, Lopez CM, Stefanidis D. Evaluating the impact of simulation on translational patient outcomes. Simul Healthc. 2011;6 Suppl:S42–S47. | |
Mariano ER, Marshall ZJ, Urman RD, Kaye AD. Ultrasound and its evolution in perioperative regional anesthesia and analgesia. Best Pract Res Clin Anaesthesiol. 2014;28(1):29–39. | |
Sites BD, Chan VW, Neal JM, et al. The American Society of Regional Anesthesia and Pain Medicine and the European Society of Regional Anaesthesia and Pain Therapy Joint Committee recommendations for education and training in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2009;34(1):40–46. | |
Woodworth GE, Chen EM, Horn JL, Aziz MF. Efficacy of computer-based video and simulation in ultrasound-guided regional anesthesia training. J Clin Anesth. 2014;26(3):212–221. | |
Udani AD, Macario A, Nandagopal K, Tanaka MA, Tanaka PP. Simulation-based mastery learning with deliberate practice improves clinical performance in spinal anesthesia. Anesthesiol Res Pract. 2014;2014:659160. | |
Niazi AU, Haldipur N, Prasad AG, Chan VW. Ultrasound-guided regional anesthesia performance in the early learning period: effect of simulation training. Reg Anesth Pain Med. 2012;37(1):51–54. | |
Moore DL, Ding L, Sadhasivam S. Novel real-time feedback and integrated simulation model for teaching and evaluating ultrasound-guided regional anesthesia skills in pediatric anesthesia trainees. Paediatr Anaesth. 2012;22(9):847–853. | |
Gasko J, Johnson A, Sherner J, et al. Effects of using simulation versus CD-ROM in the performance of ultrasound-guided regional anesthesia. AANA J. 2012;80(4 Suppl):S56–S59. | |
Garcia-Tomas V, Schwengel D, Ouanes JP, Hall S, Hanna MN. Improved residents’ knowledge after an advanced regional anesthesia education program. Middle East J Anaesthesiol. 2014;22(4):419–427. | |
Friedman Z, Siddiqui N, Katznelson R, Devito I, Bould MD, Naik V. Clinical impact of epidural anesthesia simulation on short- and long-term learning curve: high- versus low-fidelity model training. Reg Anesth Pain Med. 2009;34(3):229–232. | |
Baranauskas MB, Margarido CB, Panossian C, Silva ED, Campanella MA, Kimachi PP. Simulation of ultrasound-guided peripheral nerve block: learning curve of CET-SMA/HSL anesthesiology residents. Rev Bras Anestesiol. 2008;58(2):106–111. | |
Ouanes JP, Schwengel D, Mathur V, Ahmed OI, Hanna MN. Curriculum development for an advanced regional anesthesia education program: one institution’s experience from apprenticeship to comprehensive teaching. Middle East J Anaesthesiol. 2014;22(4):413–418. | |
Liu Y, Glass NL, Glover CD, Power RW, Watcha MF. Comparison of the development of performance skills in ultrasound-guided regional anesthesia simulations with different phantom models. Simul Healthc. 2013;8(6):368–375. | |
Kim SC, Hauser S, Staniek A, Weber S. Learning curve of medical students in ultrasound-guided simulated nerve block. J Anesth. 2014;28(1):76–80. | |
Cheung JJ, Chen EW, Al-Allaq Y, et al. Acquisition of technical skills in ultrasound-guided regional anesthesia using a high-fidelity simulator. Stud Health Technol Inform. 2011;163:119–124. | |
Bretholz A, Doan Q, Cheng A, Lauder G. A presurvey and postsurvey of a web- and simulation-based course of ultrasound-guided nerve blocks for pediatric emergency medicine. Pediatr Emerg Care. 2012;28(6):506–509. | |
Brenner GJ, Newmark JL, Raemer D. Curriculum and cases for pain medicine crisis resource management education. Anesth Analg. 2013; 116(1):107–110. | |
Morse J, Terrasini N, Wehbe M, et al. Comparison of success rates, learning curves, and inter-subject performance variability of robot-assisted and manual ultrasound-guided nerve block needle guidance in simulation. Br J Anaesth. 2014;112(6):1092–1097. | |
Lee RA, van Zundert TC, van Koesveld JJ, et al. Evaluation of the Mediseus epidural simulator. Anaesth Intensive Care. 2012;40(2):311–318. | |
Ullrich S, Frommen T, Rossaint R, Kuhlen T. Virtual reality-based regional anaesthesia simulator for axillary nerve blocks. Stud Health Technol Inform. 2009;142:392–394. | |
Sparks S, Evans D, Byars D. A low cost, high fidelity nerve block model. Crit Ultrasound J. 2014;6(1):12. | |
Rosenberg AD, Popovic J, Albert DB, et al. Three partial-task simulators for teaching ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2012;37(1):106–110. | |
Niazi AU, Ramlogan R, Prasad A, Chan VW. A new simulation model for ultrasound-aided regional anesthesia. Reg Anesth Pain Med. 2010; 35(3):320–321. | |
Liu Y, Glass NL, Power RW. Technical communication: new teaching model for practicing ultrasound-guided regional anesthesia techniques: no perishable food products! Anesth Analg. 2010;110(4):1233–1235. | |
Lim MW, Burt G, Rutter SV. Use of three-dimensional animation for regional anaesthesia teaching: application to interscalene brachial plexus blockade. Br J Anaesth. 2005;94(3):372–377. | |
Kessler J, Moriggl B, Grau T. Ultrasound-guided regional anesthesia: learning with an optimized cadaver model. Surg Radiol Anat. 2014; 36(4):383–392. | |
Inoue S, Fujiwara A, Watanabe K, Hashizume K, Furuya H. Inexpensive phantom for fluoroscopy guided nerve block training. Pain Med. 2008;9(7):863–865. | |
Hemmerling TM, Taddei R, Wehbe M, Cyr S, Zaouter C, Morse J. Technical communication: first robotic ultrasound-guided nerve blocks in humans using the Magellan system. Anesth Analg. 2013;116(2):491–494. | |
Grottke O, Ntouba A, Ullrich S, et al. Virtual reality-based simulator for training in regional anaesthesia. Br J Anaesth. 2009;103(4):594–600. | |
Capogna G, Stirparo S, Caniggia S. Evaluation of a new training device to simulate the epidural and subarachnoid spaces for neuraxial anesthesia techniques. Minerva Anestesiol. 2013;79(4):385–390. | |
Atallah J, Fahy BG, Gibson T, Martin TW. Pain simulator can improve the training of residents and pain fellows in performing pain management procedures. Pain Physician. 2007;10(3):511–512. | |
Adhikary SD, Hadzic A, McQuillan PM. Simulator for teaching hand-eye coordination during ultrasound-guided regional anaesthesia. Br J Anaesth. 2013;111(5):844–845. | |
Hocking G, Hebard S, Mitchell CH. A review of the benefits and pitfalls of phantoms in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2011;36(2):162–170. | |
Pollard BA. New model for learning ultrasound-guided needle to target localization. Reg Anesth Pain Med. 2008;33(4):360–362. | |
Bellingham GA, Peng PW. A low-cost ultrasound phantom of the lumbosacral spine. Reg Anesth Pain Med. 2010;35(3):290–293. | |
Whittaker S, Lethbridge G, Kim C, Keon Cohen Z, Ng I. An ultrasound needle insertion guide in a porcine phantom model. Anaesthesia. 2013;68(8):826–829. | |
Neal JM, Hsiung RL, Mulroy MF, Halpern BB, Dragnich AD, Slee AE. ASRA checklist improves trainee performance during a simulated episode of local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37(1):8–15. | |
Johnson B, Herring A, Stone M, Nagdev A. Performance accuracy of hand-on-needle versus hand-on-syringe technique for ultrasound-guided regional anesthesia simulation for emergency medicine residents. West J Emerg Med. 2014;15(6):641–646. | |
Gupta RK, Lane J, Allen B, Shi Y, Schildcrout JS. Improving needle visualization by novice residents during an in-plane ultrasound nerve block simulation using an in-plane multiangle needle guide. Pain Med. 2013;14(10):1600–1607. | |
Kilicaslan A, Topal A, Tavlan A, Erol A, Otelcioglu S. Differences in tip visibility and nerve block parameters between two echogenic needles during a simulation study with inexperienced anesthesia trainees. J Anesth. 2014;28(3):460–462. | |
Cook TM, Payne S, Skryabina E, Hurford D, Clow E, Georgiou A. A simulation-based evaluation of two proposed alternatives to Luer devices for use in neuraxial anaesthesia. Anaesthesia. 2010;65(11):1069–1079. | |
Brinkmann S, Mitchell CH, Hocking G. Assessment of a single-operator real-time ultrasound-guided epidural technique in a porcine phantom. Can J Anaesth. 2012;59(3):323–324. | |
Johns J, Harrison TK, Steffel L, et al. A pilot in vitro evaluation of the “air test” for perineural catheter tip localization by a novice regional anesthesiologist. J Ultrasound Med. 2014;33(12):2197–2200. | |
Kan JM, Harrison TK, Kim TE, Howard SK, Kou A, Mariano ER. An in vitro study to evaluate the utility of the “air test” to infer perineural catheter tip location. J Ultrasound Med. 2013;32(3):529–533. | |
Mariano ER, Yun RD, Kim TE, Carvalho B. Application of echogenic technology for catheters used in ultrasound-guided continuous peripheral nerve blocks. J Ultrasound Med. 2014;33(5):905–911. | |
van Geffen GJ, Mulder J, Gielen M, van Egmond J, Scheffer GJ, Bruhn J. A needle guidance device compared to free hand technique in an ultrasound-guided interventional task using a phantom. Anaesthesia. 2008;63(9):986–990. | |
Berkenstadt H, Ziv A, Gafni N, Sidi A. The validation process of incorporating simulation-based accreditation into the anesthesiology Israeli national board exams. Isr Med Assoc J. 2006;8(10):728–733. | |
Berkenstadt H, Ziv A, Gafni N, Sidi A. Incorporating simulation-based objective structured clinical examination into the Israeli national board examination in anesthesiology. Anesth Analg. 2006;102(3):853–858. | |
Dent AW, Weiland TJ, Paltridge D. Australasian emergency physicians: a learning and educational needs analysis. Part Four: CPD topics desired by emergency physicians. Emerg Med Australas. 2008;20(3):260–266. | |
Moon TS, Lim E, Kinjo S. A survey of education and confidence level among graduating anesthesia residents with regard to selected peripheral nerve blocks. BMC Anesthesiol. 2013;13(1):16. | |
O’Sullivan O, Shorten GD, Aboulafia A. Determinants of learning ultrasound-guided axillary brachial plexus blockade. Clin Teach. 2011;8(4):236–240. | |
Ajmal M, Power S, Smith T, Shorten GD. An ergonomic task analysis of spinal anaesthesia. Eur J Anaesthesiol. 2009;26(12):1037–1042. | |
Ajmal M, Power S, Smith T, Shorten GD. Ergonomic task analysis of ultrasound-guided femoral nerve block: a pilot study. J Clin Anesth. 2011;23(1):35–41. | |
Barrington MJ, Wong DM, Slater B, Ivanusic JJ, Ovens M. Ultrasound-guided regional anesthesia: how much practice do novices require before achieving competency in ultrasound needle visualization using a cadaver model. Reg Anesth Pain Med. 2012;37(3):334–339. | |
Sites BD, Gallagher JD, Cravero J, Lundberg J, Blike G. The learning curve associated with a simulated ultrasound-guided interventional task by inexperienced anesthesia residents. Reg Anesth Pain Med. 2004;29(6):544–548. | |
Sites BD, Spence BC, Gallagher JD, Wiley CW, Bertrand ML, Blike GT. Characterizing novice behavior associated with learning ultrasound-guided peripheral regional anesthesia. Reg Anesth Pain Med. 2007; 32(2):107–115. | |
Smith HM, Kopp SL, Johnson RL, Long TR, Cerhan JH, Hebl JR. Looking into learning: visuospatial and psychomotor predictors of ultrasound-guided procedural performance. Reg Anesth Pain Med. 2012;37(4):441–447. | |
Chin KJ, Tse C, Chan V, Tan JS, Lupu CM, Hayter M. Hand motion analysis using the imperial college surgical assessment device: validation of a novel and objective performance measure in ultrasound-guided peripheral nerve blockade. Reg Anesth Pain Med. 2011;36(3):213–219. | |
Cook DA. If you teach them, they will learn: why medical education needs comparative effectiveness research. Adv Health Sci Educ Theory Pract. 2012;17(3):305–310. | |
Regional Anesthesiology and Acute Pain Medicine Fellowship Directors Group. Guidelines for fellowship training in Regional Anesthesiology and Acute Pain Medicine: Second Edition, 2010. Reg Anesth Pain Med. 2011;36(3):282–288. | |
Smith HM, Kopp SL, Jacob AK, Torsher LC, Hebl JR. Designing and implementing a comprehensive learner-centered regional anesthesia curriculum. Reg Anesth Pain Med. 2009;34(2):88–94. | |
Tan JS, Chin KJ, Chan VW. Developing a training program for peripheral nerve blockade: the “nuts and bolts”. Int Anesthesiol Clin. 2010;48(4):1–11. | |
Laschinger S, Medves J, Pulling C, et al. Effectiveness of simulation on health profession students’ knowledge, skills, confidence and satisfaction. Int J Evid Based Healthc. 2008;6(3):278–302. | |
Durham CF, Alden KR. Enhancing patient safety in nursing education through patient simulation. In: Hughes RG, editor. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville (MD); Agency for Healthcare Research and Quality (US). 2008;231–253. | |
Norman G, Dore K, Grierson L. The minimal relationship between simulation fidelity and transfer of learning. Med Educ. 2012;46(7):636–647. | |
Kilicaslan A, Topal A, Tavlan A, Erol A. A simple, feedback-based simulation model for ultrasound-guided regional anaesthesia. Indian J Anaesth. 2012;56(4):431–433. | |
Ramlogan R, Manickam B, Chan VW, et al. Challenges and training tools associated with the practice of ultrasound-guided regional anesthesia: a survey of the American Society of Regional Anesthesia and Pain Medicine. Reg Anesth Pain Med. 2010;35(2):224–226. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.