Back to Journals » International Journal of Women's Health » Volume 6
Seroprevalence of human papillomavirus immunoglobulin G antibodies among women presenting at the reproductive health clinic of a university teaching hospital in Nigeria
Authors Aminu M, Gwafan JZ, Inabo HI, Oguntayo AO, Ella EE, Koledade AK
Received 22 October 2013
Accepted for publication 13 January 2014
Published 13 May 2014 Volume 2014:6 Pages 479—487
DOI https://doi.org/10.2147/IJWH.S56388
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Video abstract presented by M Aminu.
Views: 850
M Aminu,1 JZ Gwafan,1 HI Inabo,1 AO Oguntayo,2 EE Ella,1 AK Koledade2
1Department of Microbiology, Faculty of Science, Ahmadu Bello University, 2Department of Obstetrics and Gynaecology, Ahmadu Bello University Teaching Hospital, Zaria, Nigeria
Background: Human papillomavirus (HPV) is the cause of 90%–95% of squamous cell cancers. Persistent infection with high-risk HPV can lead to development of precancerous lesions of the cervix in 5%–10% of infected women, and can progress to invasive cervical cancer 15–20 years later. This study was conducted to determine the seroprevalence of HPV immunoglobulin G (IgG) antibodies among women of reproductive age attending a reproductive health clinic at Ahmadu Bello University Teaching Hospital, Zaria, Nigeria.
Methods: The study was descriptive, cross-sectional, and experimental, combining the use of a structured questionnaire and analysis of serum samples obtained from 350 consecutive consenting women. The serum samples were analyzed for IgG antibodies to HPV by enzyme-linked immunosorbent assay.
Results: We found a seroprevalence of 42.9% (150/350) for IgG antibodies to HPV in these women. Women aged 45–49 years and those who had their sexual debut aged 20–23 years had the highest HPV seroprevalence, ie, 50% (57/114) and 51.1% (46/90), respectively. Presence of antibodies varied according to sociodemographic factors, but was significantly associated with educational status, tribe, and religion (P<0.05). Human papillomavirus infection was not significantly associated with the reproductive characteristics and sexual behavior of the women. Antibodies to HPV were detected in 50.0% (9/18) of women with a family history of cervical cancer and in 30.8% (4/13) of those with a history or signs of WHIM (warts, hypogammaglobulinemia, immunodeficiency, myelokathexis) syndrome as a genetic disorder (P>0.05).
Conclusion: Further studies are needed to determine the HPV serotypes and evaluate the risk of natural development of HPV-related malignancies among women in the study area.
Keywords: seroprevalence, immunoglobulin G antibodies, human papillomavirus, women, Nigeria
Introduction
Human papillomavirus (HPV) is a non-enveloped deoxyribonucleic acid (DNA) virus belonging to the family Papillomaviridae. This family includes more than 130 genotypes,1 many of which infect the mucosal areas of the human upper digestive tract and the anogenital region through sexual contact,2,3 leading to increased risk of development of cancer.1 These genotypes are grouped into “high-risk” and “low-risk” according to the degree of risk of development of cancer after infection. Infection with the high-risk serotypes of HPV can lead to cervical cancer and are associated with other mucosal anogenital, and head and neck cancers.4,5 Infection with the low-risk serotypes is known to cause benign or low-grade cervical tissue changes and genital warts (condyloma acuminata) on the cervix, vagina, vulva, and anus in women and on the penis, scrotum, and anus in men.6,7
Genital HPV infection is one of the most common sexually transmitted infections in sexually active adolescents and young women.8,9 It has been estimated that at least 50% of sexually active adults have had a genital HPV infection2,9 and that globally 75% of individuals (males and females) will experience an HPV infection at least once in their lifetime, with the highest rates of infection occurring in those under the age of 25 years.10 In a recent meta-analysis, a global HPV prevalence of 11.7% was reported. The HPV prevalence in North America and Europe was estimated at 11.5% and 14.2%, respectively, while the prevalence in Africa was estimated at 21.1%, with sub-Saharan Africa topping the list at 24%.11,12 In Nigeria, the prevalence of HPV is high in all female age groups, and highest in women aged 15–23 years.12,13
Studies have indicated that high-risk HPV genital infections in young females are transient and have little long-term significance.2,14,15 However, when the infection persists, as in 5%–10% of infected women, there is a high risk of developing a precancerous lesion of the cervix, which can progress to invasive cervical cancer 15–20 years later.4,16–22 Persistent infection following acquisition of a high-risk HPV is generally defined by continued detection of cervical DNA of the same HPV type.23
Cervical cancer is an important health problem worldwide, being the second most common cancer among women, and ranking first in many developing countries.9,17 Half a million women develop cervical cancer annually and more than half die from the disease.9 In 2008, more than 270,000 women died of cervical cancer worldwide, with nearly 85% of these deaths occurring in developing countries.13,24 Cervical cancer is the second most common cancer in women aged 15–44 years in Nigeria and the incidence rate is 27/100,000.13,24 Current estimates indicate that every year 14,089 women are diagnosed with cervical cancer and 8,240 die from the disease in Nigeria.13 A prevalence of 26.3% for HPV in the general population has been reported in Southern Nigeria.25 The incidence of HPV in women with cervical cancer is reported to be 24.8%,12,24 while HPV prevalence in the general population (among women with normal cytology) is 23.7%.13 Risk factors associated with HPV infection include heterosexuality, promiscuity, smoking,26,27 high parity, early sexual debut,3 infection with other sexually transmitted diseases,26 prolonged use of contraceptives, dietary factors, and genetic disorders such as WHIM (warts, hypogammaglobulinemia, immunodeficiency, myelokathexis) syndrome.28,29
Infection with HPV, diagnosed by detection of antibodies to HPV in the serum or detection of HPV DNA, is the primary risk factor contributing to development of cervical intraepithelial neoplasia and invasive cervix carcinoma. Detection of anti-HPV has been shown to reflect the overall HPV infection rate in a population more effectively than detection of HPV DNA.3 This study was conducted in an area where the seroprevalence of HPV has not as yet been reported.
Materials and methods
Study area and population
The study population comprised 350 women of reproductive age (15–49 years) attending the reproductive health clinic at Ahmadu Bello University Teaching Hospital, Shika, Zaria, Nigeria. This clinic offers counseling and contraceptive services to delayers, spacers, and limiters. Post abortion care, endometrial biopsy, Papanicolaou smear, cervical punch biopsy, and colposcopy are other services offered. After obtaining ethical approval from the hospital’s ethics committee, the women were recruited and counseled, and those who gave their consent were enrolled in the study. Women who were pregnant, those who refused to give consent, and those not of reproductive age were not enrolled.
Sample collection and processing
A 3 mL blood sample was collected by venipuncture from each of the 350 women with the assistance of a laboratory scientist under the supervision of a physician. The blood samples were transported immediately to the laboratory in the Department of Microbiology, Faculty of Science, Ahmadu Bello University, Zaria, which is located 5 km from the hospital. The blood samples were allowed to clot for 30 minutes and then centrifuged at 1,000× g for 10 minutes to separate the serum. The serum samples were stored at −20°C until analysis.
Detecting of IgG antibodies to HPV
An enzyme-linked immunosorbent assay (Wkea Med Supplies Corporation, Changchun, People’s Republic of China) was used to screen for HPV immunoglobulin G (IgG) antibodies in the serum samples. The test was carried out according to the manufacturer’s instructions. All reagents were brought to room temperature and mixed thoroughly by gentle swirling before use. The cutoff value was calculated using the manufacturer’s specifications. The average optical density value of the negative control wells plus 0.15 was taken as the cutoff value. A negative HPV result was interpreted as any sample with an optical density value less than the calculated cutoff value, and samples with an optical density greater than the calculated cutoff value were reported as positive for IgG to HPV.
Data analysis
The results and data from the questionnaires were analyzed, reduced to percentages, and presented as tables and figures. The statistical analysis was done using Statistical Package for the Social Sciences version 17 software (SPSS Inc., Chicago, IL, USA). Associations between variables were identified by Pearson’s chi-square analysis and relationships between HPV and risk factors were identified by Spearman’s rank correlation. Two-tailed P-values <0.05 were considered to be statistically significant.
Results
Sociodemographics, reproductive characteristics, and sexual behavior
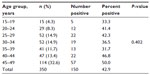
The sociodemographic data for the women obtained from the questionnaires were analyzed and the results showed that women aged 45–49 years were most frequently seen (32.6%, 114/350) in the clinic while those aged 15–19 years were the least often seen (4.3%, 15/350, Table 1). Further analysis showed that 38.0% (133/350) of the women were from tribes other than the three major Nigerian ethnic groups. Further, 39.7% (139/350) were civil servants, 65.1% (228/350) were married, 56% (199/350) were in monogamous marriages, and 4.3% (15/350) admitted to smoking (Table 2).
  | Table 1 Seroprevalence of human papillomavirus immunoglobulin G antibodies according to age group |
  | Table 2 Seroprevalence of immunoglobulin G antibodies to human papillomavirus according to sociodemographic characteristics |
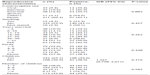
The reproductive characteristics and sexual behavior of the women are shown in Tables 3 and 4, respectively. The data show that 54.9% (192/350) of the women had been married for less than 25 years and 56.3% (197/350) had had less than seven successful pregnancies (parity), ie, reaching at least 28 weeks’ gestation. In total, 69.1% (242/350) of the women had children, with 57.1% (200/350) having 1–6 children. Further analysis of the data showed that 60.3% (211/350) of the women had never used any form of contraception, and a sizeable proportion (3.1%, 11/350) had used other forms or non-modern contraception (Table 3). The most frequent age of sexual debut was ≤19 years (26.9%, 94/350) while 8.9% (31/350) had their first child aged 15–18 years (Table 4).
  | Table 3 Distribution of human papillomavirus according to reproductive characteristics |
  | Table 4 Seroprevalence of immunoglobulin G antibodies to human papillomavirus according to sexual behavior and genital complaints |
Analysis of enzyme-linked immunosorbent assay
Human papillomavirus IgG antibodies were detected in serum samples from 42.9% (150/350) of women. Antibodies to HPV initially increased with age, decreased in women aged 30–39 years, and then increased again from age 40 years, peaking in women aged 45–49 years (50.0%, 57/114). Women aged 35–39 years had the lowest prevalence (31.7%, 13/41, Table 1). The HPV infection rate did not differ significantly according to patient age (χ2=6.192, df =6, P=0.402).
The seroprevalence of HPV according to other demographic characteristics is shown in Table 2. Women belonging to tribes other than the three major Nigerian ethnic groups had the highest HPV prevalence (44.0%, 66/119), while Igbo women had the lowest prevalence (4.7%, 7/25). There was a marginal association between HPV infection rate and tribal origin (χ2=7.595, df =3, P=0.05).
HPV antibodies were detected significantly (χ2=3.938, df =1, P=0.03) more often in Christian women (47.1%, 99/203) than in Muslim women (36.4%, 51/140). Retired women had the highest prevalence (75.0%, 9/12) and unemployed women had the lowest prevalence (35.1%, 20/57); however, this finding was not statistically significant (χ2=7.820, df =4, P=0.098).
The seroprevalence of HPV was highest among widows (53.1%, 17/32) and lowest among divorcees (35.7%, 5/14), but the difference was not statistically significant (χ2=1.739, df =3, P=0.773). The seroprevalence of HPV IgG antibodies was similar between monogamous (43.2%, 86/199) and polygamous women (42.7%, 32/75; χ2=0.007, df =1, P=0.935; odds ratio 1.023; 95% confidence interval 0.598–1.749).
HPV infection was significantly associated with educational status (χ2=6.594, df =4, P=0.043). Antibodies were detected with the highest prevalence among women with secondary education (49.2%, 29/59) while those with Quranic education had the lowest prevalence (21.7%, 5/23, Figure 1). Antibodies to HPV were detected with a slightly higher frequency (43.1%, 140/325) in women who were not smoking compared with those who admitted to smoking (40.0%, 10/25; χ2=0.090, df=1, P=0.765).
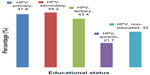  | Figure 1 Seroprevalence of human papillomavirus immunoglobulin G antibodies according to educational status (χ2=6.594, df=4, P=0.043). |
The seroprevalence of HPV according to reproductive characteristics is shown in Table 3. Analysis of the results according to years of marriage showed that the highest frequency of antibodies to HPV was among women who had been married for 16–20 years (55.3%, 21/38) and the lowest frequency in those who had been married for 26–30 years (28.6%, 6/21; χ2=8.734, df =7, P=0.262).
No statistically significant relationship was found between seroprevalence of HPV and parity (χ2=3.700, df =4, P=0.448), but the prevalence was highest amongst women who had 7–9 pregnancies (46.7%, 21/45) and lowest in those of parity >9 (25.0%, 3/12).
Women with children had a higher prevalence of antibodies to HPV (44.6%, 108/242) than those without children (38.9%, 42/108). Although the difference observed was not statistically significant (χ2=1.004, df =1, P=0.316), women with children were 1.3 times more likely to be infected than women without children (odds ratio 1.267; 95% confidence interval 0.798–2.011). Women who had 7–9 children had the highest seroprevalence (46.9%, 15/32) while those with more than nine children had the lowest seroprevalence (16%, 1/6; χ2=2.851, df =4, P=0.583).
The seroprevalence of IgG antibodies to HPV was not significantly associated with use of contraceptives (χ2=1.169, df =4, P=0.883); women who were using other types of contraception (eg, not modern or local contraceptives) had the highest prevalence (54.5%, 6/11) while those using oral contraception had the lowest prevalence (39.4%, 13/33).
The seroprevalence of HPV according to sexual behavior is shown in Table 4. Antibodies to HPV were detected most frequently among women who had their sexual debut aged 20–23 years (51.1%, 46/90) and least often in women who had their sexual debut aged 24–26 years (37.9%, 25/66). However, this difference was not statistically significant (χ2=3.114, df =5, P=0.682). The frequency of detection of IgG antibodies to HPV according to age at birth of first child was highest among women older than 30 years (68.8%, 11/16) and lowest among women aged 15–18 years (35.5%, 11/31; χ2=8.163, df =5, P=0.147).
The prevalence of HPV was also investigated according to vaginal symptoms reported by the women during the study. Women with normal vaginal discharge had the highest frequency of HPV antibodies (45.1%, 110/244) while women with abnormal discharge (37.7%, 40/106) had the lowest frequency (χ2=1.628; df =1; P=0.202). Further, women with genital rash at the time of the study had the highest seroprevalence (61.9%, 13/21), with the lowest seroprevalence seen in women with vaginal itching (33.3%, 26/78). The difference in prevalence observed according to vaginal symptoms was almost statistically significant (χ2=7.461, df =3, P=0.059).
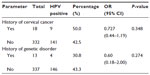
Half of the women (50.0%, 9/18) with a family history of cervical cancer had IgG antibodies to HPV compared with those without a family history of cervical cancer (42.5%, 141/332; χ2=0.395, df =1, P=0.348). Antibodies to HPV tended (χ2=0.806; df =1; P=0.274) to be detected with a higher prevalence in women without a history or signs of WHIM as a genetic disorder (43.3%, 146/337) than in those with such a history (30.8%, 4/13, Table 5).
Discussion
In this study, HPV IgG antibodies were detected in serum samples from 150 of 350 women studied, giving a seroprevalence of 42.9%. This percentage is higher than the 26.3% reported in Ibadan, Nigeria25 and similar to the 40% reported in southern Mozambique.31 The higher seroprevalence in the present study compared with that in a previous report from Nigeria may be due to a difference in the study population. Another reason could be the high sensitivity of the enzyme-linked immunosorbent assay that permits detection of the HPV antibody in samples with low HPV antibody which would probably otherwise have been scored as negative for HPV. It has been shown that detection of antibodies to HPV can evaluate the overall HPV infection rate in a population more effectively than HPV DNA.3 In view of this, and because Ahmadu Bello University Teaching Hospital is a referral center where patients from Zaria and its environs are seen, and 40% of the women attending the clinic there have been infected with HPV, this percentage represents the actual seroprevalence of HPV in the state.
This high seroprevalence could be due to the lifestyle of the local population in the study area, where women are constantly exposed to the virus, by means of early sexual debut, early marriage, multiple sexual partners due to polygamy, and high divorce rates. Acquisition of HPV infection has been shown to be strongly related to sexual behavior, and the prevalence of HPV increases with increasing number of sexual partners and early sexual debut.3,30
About one third of the women attending the reproductive health clinic were aged 45–49 years, also the group in which HPV was most prevalent. The detection of HPV in older women in this study is consistent with a previous report,32 and could be due to the sexual mode of transmission of the virus which allows reinfection and persistence of the virus for years.33,34 These women might have acquired the virus at an earlier age, considering that the virus has been shown to persist in a significant percentage of women.20,22
Antibodies to HPV were also detected in younger women in this study, probably because of early acquisition of infection as a result of early indulgence in sexual activity and early marriage. Women in the study area often marry as young as age 15 years, and it has been reported that the age of sexual debut in Nigeria is 9–10 years.35 However, detection of antibodies to HPV, which signifies infection, does not mean eventual development of cervical cancer. This is because most HPV infections in younger women are transient or asymptomatic, often spontaneously regress,36 and have little long-term significance. Moreover, the incidence of cervical cancer in women younger than 30 years is very low, and 70% of cases of HPV infection resolve in one year and 90% in 2 years.22,37 However, persistent infection with one or more high-risk types of HPV is an important etiologic factor in the development of cervical intraepithelial neoplasia and progression to cervical cancer.20,22,38–40 In addition, virologic, environmental, immunologic, and genetic factors have also been implicated in the development of cervical cancer.41
The prevalence of HPV according to occupational status of the woman was not statistically significant in this study. This means that all women, regardless of occupation were at similar risk of being infected. Single and married women in this study had a similar seroprevalence of HPV, indicating a similar rate of sexual activity. The seroprevalence of HPV was higher in widows than in other studies, which reported a higher prevalence among married women.31,39 Yet other studies have reported the highest prevalence among single women.33,41 The prevalence of antibodies to HPV was similar between women who married into monogamous homes and those who married into polygamous homes. This study presents a paradoxical picture that is in contrast with the widely held belief that sexual activity in individuals with multiple partners increases the risk of HPV infection. Monogamy does not necessarily mean adhering to a single partner.
It has been shown that educational level is a socioeconomic factor with an effect on risk of HPV infection. More educated women had the highest infection rate in our study, with the highest prevalence seen in women with secondary school education. This result is consistent with previous research,32,39 but in contrast with a report by Marrazzo et al42 who showed that the HPV infection rate decreased with increasing level of education. This observation could be due to early indulgence in sexual activity and lack of awareness of its consequences.
Smoking has been established as one of the major risk factors for HPV infection. However, in our study, HPV was not significantly associated with smoking and was detected with a higher prevalence among nonsmokers. This observation contrasts that of Schlecht et al.39 An earlier study reported that daily cigarette smoking had a deleterious effect and contributed to development of low-grade squamous intraepithelial lesions.43 Several epidemiologic studies have identified a role of cigarette smoking in invasive cervical cancer.26,27,44 Nicotine and other cigarette metabolites have been found in cervical mucus.45 It is also suspected that the relationship between cigarette smoking and low-grade squamous intraepithelial lesions reflects a link between smoking and immune dysregulation,46 and it has been suggested that smoking may induce an impaired antibody response in young women infected with HPV16/18.27
Studies have shown that use of oral contraceptives is a risk factor for acquiring HPV, and prolonged use of oral contraceptives is associated with development of squamous intraepithelial lesions.29,47 In this study, even though, HPV antibodies were detected with the highest prevalence among women who used non-modern contraceptives compared with those using modern contraceptives, the difference in prevalence was however, not statistically significant. This result contrasts with previous reports.33,39 Other studies have recorded a higher prevalence of HPV infection in women who do not use any form of contraception.32,42 However, a recent study conducted in Kano, Nigeria, did not find a significant association between use of oral contraceptives and HPV infection.30
Number of years of marriage had no significant effect on infection with HPV in this study, implying that HPV can be acquired at any time during marriage. Parity also had no significant effect on HPV infection in this study. Women with high parity had the least prevalence, as previously reported.39 Low parity was recently reported to be a significant risk factor for acquisition of HPV in northern Nigeria.30 In contrast, Okolo et al,25 in southern Nigeria, observed that the prevalence of HPV increases with increasing parity. This increase in prevalence of HPV infection with increasing parity has been attributed to increased sexual activity.3,34
In our study, women who had children had a higher prevalence of antibodies to HPV than women with no children. Although this difference in prevalence did not reach statistical significance, women who had children were 1.3 times more likely to be infected with HPV than those who did not. This observation is consistent with a report by Firnhaber et al.48 It was also observed that antibodies to HPV were detected more often in women who had had multiple pregnancies than in women who had never been pregnant. A similar observation was made by Trottier et al,34 who also agreed with the view that parity is a risk factor for acquisition of HPV infection. According to the American Cancer Society,49 women who have had three or more full-term pregnancies have an increased risk of developing cervical cancer due to having unprotected intercourse to become pregnant, so they may have had more exposure to HPV. Further, studies have pointed to hormonal changes during pregnancy as a possible factor making women more susceptible to HPV infection or development of cancer.49
Women in our study who had their sexual debut aged 20–23 years had the highest prevalence of HPV antibodies. This agrees with previous reports of an increased HPV among women who indulge in sexual activity at an early age.25,30,32,41 Acquisition of HPV infection is strongly associated with sexual behavior. The prevalence of HPV increases with number of sexual partners and earlier sexual debut.3 Antibodies to HPV were detected more often in women who had had their first child at age older than 30 years. In 2012, the American Cancer Society reported that women who were younger than 17 years when they had their first pregnancy were almost twice as likely to develop cervical cancer later in life than women who did not become pregnant until they were aged 25 years or older.49
Abnormal discharge was found in 37.7% of our women, although this finding was not statistically significant. Abnormal discharge is the commonest sign observed when there is infection in the reproductive organs.50 Discharge is always seen when neoplasia is present and in the advanced stages of HPV infection.51 The majority of the women attending our reproductive health clinic had normal vaginal discharge. Abnormal vaginal symptoms included itching, rash, and ulcer, with vaginal itching being the most common (P=0.059). Half of the women with a family history of cervical cancer had antibodies to HPV. Cervical cancer may run in some families. If cervical cancer is known in a family to be genetic, the chances of developing the disease are 2–3 times higher than if no one in the family has it.49 Some researchers suspect that in some cases this familial tendency is caused by an inherited condition that makes some women less able to fight off HPV infection than others.49 Four of the 13 women who had a history of or some signs of WHIM as a genetic disorder had antibodies to HPV, but the antibodies were not detected with a statistically significance difference. It has however been reported that the main clinical problem of patients with WHIM, is unusual susceptibility to infection with HPV51,52 and the most common signs are repeated bacterial infections, neutropenia and extensive infection with HPV leading to dermal and genital warts.52
Conclusion
HPV IgG antibodies were detected in 42.9% of the women enrolled in this study, indicating that the women had been infected and that the virus is circulating with a high prevalence in Kaduna State. Infection with HPV is a major risk factor contributing to the development of cervical intraepithelial neoplasia and invasive cervical carcinoma. Women aged 45–49 years and those who had their sexual debut when aged 20–23 years had the highest seroprevalence of HPV. Infection with HPV varied with the women’s reproductive characteristics, sexual behavior, and sociodemographic factors but did not reach statistically significant levels except for educational status, tribe, and religion.
Recommendations
In view of the high prevalence of antibodies to HPV detected in this study, which denotes a high infection rate in the area, we recommend introduction of a subsidized HPV vaccine in our national immunization schedule in other to prevent the current scourge of cervical cancer in Nigeria. Two prophylactic vaccines, ie, Gardasil® (Merck and Co, Inc., Whitehouse Station, NJ, USA) and Cervarix® (GlaxoSmithKline, London, UK), that are safe and effective against anogenital HPV,53 are currently available for the prevention of genital HPV infection.1
In view of the results of the present study and those of another recent study showing low knowledge of HPV and its vaccines among Nigerian mothers with a high awareness for cervical cancer but little knowledge of its link to HPV,12 we recommend improving knowledge at the population level by education on the mode of transmission of the virus and the risks associated with the virus as a cause of cervical cancer.
In addition, cervical cancer screening programs need to be put in place in Nigeria, given that cervical cancer has been shown to disproportionately affect African American women, who are nearly twice more likely than European American women to die of the disease.22,54 It is important to screen women for anti-HPV, and if positive, detect the genotypes. Those found to be infected with the high-risk genotypes should have Papanicolaou screening annually in order to prevent cervical cancer.
Study limitations
The majority of the women who took part in this study could not communicate in the English language and used an interpreter, so some of the data might not accurately reflect their sociodemographic, reproductive characteristics, or sexual behavior. In addition, some were not willing to disclose all information concerning their reproductive characteristics and sexual behavior. Also, we were not able to determine the presence of HPV DNA in HPV IgG-positive serum.
Acknowledgment
We acknowledge all the women who took part in the study and all the personnel from the reproductive health clinic at Ahmadu Bello University Teaching Hospital, Zaria, Nigeria, for their cooperation with collection of samples.
Disclosure
The authors report no conflicts of interest in this work.
References
Frazer IH. Measuring serum antibody to human papillomavirus following infection or vaccination. Gynecol Oncol. 2010;118(Suppl 1):S8–S11. | |
Touzé A, de Sanjosé S, Coursaget P, et al. Prevalence of anti-human papillomavirus types 16, 18, 31, and 58 virus-like particles in women in the general population and in prostitutes. J Clin Microbiol. 2001;39:4344–4348. | |
Ma GX, Wang MQ, Ma XS, Shive SE, Tan Y, Toubbeh JI. Pathways of cervical cancer screening among Chinese women. Int J Womens Health. 2013;5:351–359. | |
Bosch FX, Manos MM, Muñoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. | |
Chew GK, Cruickshank ME, Rooney PH, Miller ID, Parkin DE, Murray GI. Human papillomavirus 16 infection in adenocarcinoma of the cervix. Br J Cancer. 2005;93:1301–1304. | |
Palefsky JM. Serological detection of human papillomavirus-related anogenital disease: new opportunities and challenges. J Natl Cancer Inst. 1995;87:437–440. | |
Centers for Disease Control and Prevention. Genital HPV Infection – CDC Fact Sheet. Available from: http://www.cdc.gov/std/HPV/STDFact-HPV.htm.2009. Accessed May 14, 2012. | |
Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific HPV infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485–490. | |
Di Giuseppe G, Abbate R, Liguori G, Albano L, Angelillo IF. Human papillomavirus and vaccination: knowledge, attitudes, and behavioural intention in adolescents and young women in Italy. Br J Cancer. 2008;99:225–229. | |
Trim K, Nagji N, Elit L, Roy K. Parental knowledge, attitudes, and behaviours towards human papillomavirus vaccination for their children: a systematic review from 2001 to 2011. Obstet Gynecol Int. 2012;2012:921236. | |
Dahlstrom LA, Tran TN, Lundholm C, Young C, Sundström K, Sparén P. Attitudes to HPV vaccination among parents of children aged 12–15 years – a population-based survey in Sweden. Int J Cancer. 2010;126:500–507. | |
Ezenwa BN, Balogun MR, Okafor IP. Mothers’ human papilloma virus knowledge and willingness to vaccinate their adolescent daughters in Lagos, Nigeria. Int J Womens Health. 2013;5:371–377. | |
Bruni L, Barrionuevo-Rosas L, Serrano B et al. ICO Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Nigeria. Summary Report March 17, 2014. Accessed March 20, 2014. | |
Hildesheim A, Schiffman MH, Gravitt PE, et al. Persistence of type specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–240. | |
Hinchliffe SA, van Velzen D, Korporaal H, Kok PL, Boon ME. Transience of cervical HPV infection in sexually active young women with normal cervicovaginal cytology. Br J Cancer. 1995;72:943–945. | |
Bosch FX, Lorincz A, Meijer CJ, Shah KV. The causal relationship between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. | |
Rock CL, Michael CW, Reynolds RK, Ruffin MT. Prevention of cervix cancer. Crit Rev Oncol Hematol. 2000;33:169–185. | |
Moscicki A, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. | |
Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. 2009;113:18–25. | |
Goodman MT, Shvetsov YB, McDuffie K, et al. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res. 2008;68:8813–8824. | |
Fukui A, Matsueda S, Kawano K, et al. Identification of B cell epitopes reactive to human papillomavirus type-16L1-derived peptides. Virol J. 2012;9:199. | |
Banister CE, Messersmith AR, Chakraborty H, et al. HPV prevalence at enrolment and baseline results from the Carolina Women’s Care Study, a longitudinal study of HPV persistence in women of college age. Int J Womens Health. 2013;5:379–388. | |
Cuschieri KS, Cubie HA, Whitley MW. Persistent high risk HPV infection associated with development of cervical neoplasia in a prospective population study. J Clin Pathol. 2005;58:946–950. | |
Nnodu O, Erinosho L, Jamda M, et al. Knowledge and attitudes towards cervical cancer and human papillomavirus: a Nigerian pilot study. Afr J Reprod Health. 2010;14:95–108. | |
Okolo C, Fianceschi S, Adewole I, et al. Human papillomavirus infection in women with and without cervical cancer in Ibadan, Nigeria. Infect Agent Cancer. 2010;1:24. | |
Matsumoto K, Yasugi T, Oki A, et al. Are smoking and chlamydial infection risk factors for CIN? Different results after adjustment for HPV DNA and antibodies. Br J Cancer. 2003;89:831–833. | |
Simen-Kapeu A, Kataja V, Yliskoski M, et al. Smoking impairs human papillomavirus (HPV) type 16 and 18 capsids antibody response following natural HPV infection. Scand J Infect Dis. 2008;40:745–751. | |
Kwasniewska A, Charzewska J, Tukendorf A, Semczuk M. Dietary factors in women with dysplasia coli of uteri associated with human papillomavirus infection. Nutr Cancer. 1998;30:39–45. | |
Thomas JO, Herrero R, Omigbodun AA, Ojemakinde K, Ajayi IO, Fawole A. Prevalence of papillomavirus infection in women in Ibadan, Nigeria: a population-based study. Br J Cancer. 2004;90:638–645. | |
Auwal IK, Aminu M, Atanda AT, Tukur J, Sarkinfada F. Prevalence and risk factors of high risk human papillomavirus infections among women attending gynaecology clinics in Kano, Northern Nigeria. Bayero Journal of Pure and Applied Sciences. 2013;6:67–71. | |
Menéndez C, Castellsagué X, Renom M, et al. Prevalence and risk factors of sexually transmitted infections and cervical neoplasia in women from a rural area of southern Mozambique. Infect Dis Obstet Gynecol. 2010;2010:pii:609315. | |
Naucler P, Chen HC, Persson K, et al. Seroprevalence of human papillomaviruses and Chlamydia trachomatis and cervical cancer risk: nested case-control study. J Gen Virol. 2007;88:814–822. | |
Sellors JW, Karwalajtys TL, Kaczorowski J, et al. Incidence, clearance and predictors of human papillomavirus infection in women for the survey of HPV in Ontario Women. CMAJ. 2003;168:421–425. | |
Trottier H, Mahmud S, Prado JC, et al. Type-specific duration of human papillomavirus infection: implications for human papillomavirus screening and vaccination. J Infect Dis. 2008;197:1436–1447. | |
Kolawole AB. Cervical cancer and its control in Nigeria: challenges and the way forward. Presented at the 44th International Course in Health and Development, Royal Tropical Institute, Amsterdam, The Netherlands, September 12, 2008. | |
Cuzick J, Beverley E, Ho L. HPV testing in primary screening of older women. Br J Cancer. 1999;81:554–558. | |
Goldstein MA, Goodman A, del Carmen MG, Wilbur DC. Case records of the Massachusetts General Hospital. Case 10-2009. A 23-year-old woman with an abnormal Papanicolaou smear. N Engl J Med. 2009;360:1337–1344. | |
Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. | |
Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286:3106–3114. | |
Winer RL, Kiviat NB, Hughes JP. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731–738. | |
Tábora N, Zelaya A, Bakkers J, Melchers WJ, Ferrera A. Chlamydia Trachomatis and genital human papillomavirus infections in female university students in Honduras. Am J Trop Med Hyg. 2005;73:50–53. | |
Marrazzo JM, Koutsky LA, Kiviat NB, Kuypers JM, Stine K. Papanicolaou test screening and prevalence of genital human papillomavirus among women who have sex with women. Am J Public Health. 2001;91:947–952. | |
Moscick AB, Wenberg A, Song LY. Updating the national history of HPV and anogenital safety and immunology of cancer. Vaccine. 2006;24:42–51. | |
Winkelstein W, Shillitoe EJ, Brand R, Johnson KK. Further comments on cancer of the uterine cervix, smoking, and herpes virus infection. Am J Epidemiol. 1984;119:1–8. | |
Schiffman MH, Haley NJ, Felton JS, et al. Biochemical epidemiology of cervical neoplasia measuring cigarette smoke constituents in the cervix. Cancer Res. 1987;47:3886–3888. | |
Barton SE, Maddox PH, Jenkins D, Edwards R, Cuzick J, Singer A. Effect of cigarette smoking on cervical epithelial immunity: a mechanism for neoplastic change? Lancet. 1998;2:652–654. | |
Clarke EA, Hatcher J, McKeown-Eyssen GE, Lickrish GM. Cervical dysplasia association with sexual behavior, smoking, and oral contraceptive use? Am J Obstet Gynecol. 1985;151:612–616. | |
Firnhaber C, Van Le H, Pettifor A, et al. Association between cervical dysplasia and human papillomavirus in HIV seropositive women from Johannesburg, South Africa. Cancer Causes Control. 2010;21:433–443. | |
American Cancer Society. Cervical Cancer. Available from: http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-risk-factors. Accessed March 15, 2014. | |
Rylander E, Berglund AL, Krassny C, Petrini B. Vulvovaginal candida in a young sexually active population: prevalence and association with oro-genital sex and frequent pain at intercourse. Sex Transm Infect. 2004;80:54–57. | |
Liu Q, Chen H, Ojode T, et al. WHIM syndrome caused by a single amino acid substitution in the carboxyl-tail of chemokine receptor CXCR4. Blood. 2012;120:181–189. | |
Kawai T, Malech HL. WHIM syndrome: congenital immune deficiency disease. Curr Opin Hematol. 2009;16(1):20–26. | |
Brown DR, Garland SM, Ferris DG, et al. The humoral response to Gardasil over four years as defined by total IgG and competitive Luminex immunoassay. Hum Vaccin. 2011;7:230–238. | |
American Cancer Society. Cancer Facts and Figures 2013. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. Accessed September 10, 2013. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.