Back to Journals » Clinical Ophthalmology » Volume 8
Safety and effectiveness of a single-piece hydrophobic acrylic intraocular lens (enVista®) – results of a European and Asian-Pacific study
Authors Heiner P, Ligabue E, Fan A, Lam D
Received 17 October 2013
Accepted for publication 13 January 2014
Published 27 March 2014 Volume 2014:8 Pages 629—635
DOI https://doi.org/10.2147/OPTH.S56135
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Peter Heiner,1 Edoardo Ligabue,2 Alex Fan,3 Dennis Lam3
1Vision Eye Institute, Southport, QLD, Australia; 2Ophthalmic Center, San Siro Clinic, Milan, Italy; 3Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong
Purpose: To evaluate the safety and effectiveness of a single-piece hydrophobic acrylic intraocular lens (IOL) (enVista® MX60; Bausch and Lomb Incorporated, Rochester, NY, USA) following implantation to correct aphakia subsequent to extracapsular cataract extraction in adults.
Subjects and methods: This was an open-label, non-interventional, observational study conducted in 19 university and private-practice settings in Europe and the Asia-Pacific region to investigate clinical outcomes of the MX60 IOL in standard practice. Eligible subjects were at least 18 years of age and had undergone standard phacoemulsification and extracapsular cataract extraction with implantation of the MX60 IOL. The primary safety endpoint was the occurrence of adverse events, and the primary effectiveness endpoints included visual and refractive outcomes and stability, with data collected up to 2 years post-procedure.
Results: In this multicenter study, pooled data of 255 eyes were collected and analyzed. Excellent visual and refractive outcomes and stability were demonstrated. At postoperative visit 4 (61–180 days postoperative), 62.2% of subjects achieved a Snellen best-corrected distance visual acuity (CDVA) of 20/20 (decimal 1.00), and 97.8% of subjects achieved a CDVA of 20/40 (decimal 0.50) or better. One eye (1.0%) underwent neodymium:yttrium aluminum garnet capsulotomy at 12 months post-procedure. No glistenings of any grade were reported for any subject at any visit. Adverse events were infrequent and were consistent with incidences generally reported with cataract surgery.
Conclusion: This study, which enrolled all comers, provided evidence of the excellent safety and effectiveness of the MX60 IOL in standard practice. Favorable clinical outcomes included outstanding visual and refractive outcomes and stability. No glistenings were reported at any postoperative visit.
Keywords: cataract surgery, glistenings, IOL, MX60
Introduction
Recent advances in intraocular len (IOL) material and design offer enhanced treatment options to surgeons and patients. As excellent visual and refractive outcomes have become more expected, increased awareness has focused on visual and refractive stability and on reductions in posterior capsule opacification (PCO) and glistenings. Although neodymium:yttrium aluminium garnet (Nd:YAG) capsulotomy is generally performed in-office with a high index of safety, it can result in complications.1–4
The visual significance of glistenings, fluid-filled microvacuoles that form within the IOL optic when in an aqueous environment,5 is a matter that has been actively investigated. A review of published literature on glistenings describes them in association with hydrophobic acrylic IOLs; however, the hydrophobic acrylic IOLs on the market are not manufactured from the same material and by the same manufacturing process.5 The impact of glistenings on visual performance is controversial, but there is the suspicion that severe presentations of glistenings may affect visual function.6 In any case, a pristine IOL is the preferred outcome.
The MX60 (enVista® MX60; Bausch and Lomb Incorporated, Rochester, NY, USA), an IOL in which both the optic and haptics are formed from a proprietary glistening-free hydrophobic acrylic polymer, received Conformité Européene (CE [European Conformity]) mark approval in August 2010. Following a multicenter, prospective, randomized clinical trial,7 the MX60 was approved by the US Food and Drug Administration (FDA) as a single-piece hydrophobic acrylic IOL in May 2012. To obtain further data characterizing the safety and effectiveness of this IOL, a multicenter, observational study was initiated in Europe and the Asia-Pacific region to explore real-world clinical practice outcomes.
Subjects and methods
Study design
This was an open-label, non-interventional clinical study conducted at 15 sites in Europe, two sites in Hong Kong, and two sites in Australia, involving 21 surgeon investigators. The study protocol was reviewed and approved by independent ethics committees prior to initiation of the study at each center. The study was conducted in accordance with International Conference on Harmonisation Good Clinical Practice8 and applicable local regulations. Written informed consent was obtained from subjects prior to having any study-related procedure.
The study protocol allowed flexibility in patient selection and surgical technique to reflect each investigator’s current practice of cataract surgery. The primary safety endpoint of this study was the occurrence of adverse events (AEs), described in terms of statistical incidence rates. The primary effectiveness endpoints included visual and refractive outcomes and stability.
Eligibility criteria
Subjects 18 years of age or older who underwent standard phacoemulsification/extracapsular cataract extraction with implantation of the MX60 IOL were enrolled in this study. As the intent of this study was to obtain clinical outcomes found in standard practice, there were no other specific inclusion or exclusion criteria. Both eyes of subjects were eligible for inclusion in this study.
IOL material and design
The MX60 IOL optic and haptics are lathed and milled from a single button made from a proprietary soft hydrophobic acrylic polymer, which incorporates an ultraviolet-absorbing chromophore. Surface energy and contact angle measurements have demonstrated that the IOL material is a true hydrophobic polymer with water contact angle values comparable to those of other hydrophobic acrylic IOLs.9 To ensure that the IOL remains glistening-free, this IOL is prehydrated to equilibrium water content.
The surface hardness of the material is 11.0 MPa, which compares favorably with the 0.24 MPa, 0.68 MPa, and 0.43 MPa reported for AcrySof (Alcon Laboratories, Inc., Fort Worth, TX, USA), Acryfold (Hoya Corporation, Santa Clara, CA, USA), and Sensar (Abbott Medical Optics, Abbott Park, IL, USA) IOL materials, respectively.10 The high surface hardness of the material results in a lens which is difficult to either scratch or deform. The reduced compliance of the material also results in a requirement for greater force in folding the lens for insertion into the injection cartridge.7 The material from which the MX60 lens is manufactured has a relatively high glass transition temperature (28°C), which results in slow unfolding in the eye. The lens material is highly temperature sensitive, so that slight warming will result in more rapid unfolding in the eye.11
The biconvex lens optic has a body diameter of 6.0 mm and an overall length (diameter) of 12.5 mm (Figure 1). The MX60 IOL has aberration-free aspheric optics and modified C-loop haptics.12–14 The anterior edge is designed to allow anterior capsulorhexis adherence for improved lens centration. The 360° square posterior edge is designed to minimize the development of PCO (Figure 2).15 The MX60 IOL features step-vaulted haptics that translate the optic posteriorly for direct contact with the capsular bag in addition to a sharp 360° square barrier edge to inhibit lens epithelial cell migration. In addition, the haptics have fenestrations intended to prevent transfer of stress from the haptic to the optic.
  | Figure 1 The design of the MX60 (Bausch and Lomb Incorporated, Rochester, NY, USA) intraocular lens. |
  | Figure 2 The MX60 (Bausch and Lomb Incorporated, Rochester, NY, USA) intraocular lens. The diagram shows the posterior surface facing toward the right side of the page. The haptics are offset anteriorly with respect to the optic body, which enables consistent posterior movement of lens optic under haptic compression. Image courtesy of David Spalton, FRCS, FRCP, FRCOphth. © 2013 Dove Medical Press Ltd. Reproduced with permission from Packer M, Fry L, Lavery KT, Lehmann R, et al. Safety and effectiveness of a glistening-free single-piece hydrophobic acrylic intraocular lens (enVista). Clin Ophthalmol. 2013;7:1905–1912.28 |
Study procedures
Eligible subjects who provided written informed consent had a preoperative visit, an operative visit (day 0), and up to six postoperative visits (visit 1, 1–2 days postoperative; visit 2, 3–14 days postoperative; visit 3, 15–60 days postoperative; visit 4, 61–180 days postoperative; visit 5, 181–365 days postoperative; and visit 6, 366–730 days postoperative). The preoperative visit included determinations of uncorrected distance visual acuity (UDVA), best-corrected distance visual acuity (CDVA), manifest refraction, keratometry, axial length, target postoperative refraction, and biometry.
Postoperative assessments included evaluation of UDVA, CDVA, manifest refraction, glistenings assessment through slit-lamp examination, and monitoring for AEs and secondary interventions.
Evaluation of glistenings
Glistenings, which are fluid-filled microvacuoles that can form within the IOL optic when in an aqueous environment, are more common in hydrophobic IOLs than in other lens materials.5 Glistenings assessment was performed by slit-lamp examination using high-powered magnification and were graded according to either absent or, if present, according to the following scale (grade 1, less than five glistenings observed; grade 2, more than five glistenings observed but not affecting vision; grade 3, glistenings affecting vision).
Statistical methods
Descriptive statistics were used to summarize the data. Continuous variables were summarized by the sample size, mean, standard deviation (SD), and the minimum and maximum. Categorical variables were summarized by frequency and percentage. All AEs reported at all visits were summarized cumulatively.
Results
Demographics and surgery
A total of 255 eyes were included in the pooled data analysis. The mean ± SD age of patients included in the analysis was 73.3±9.6 years. The majority of eyes (59.2%) were from female subjects, and more procedures were completed in the right eye of subjects (54.1%). At baseline, 27 eyes (10.6%) were reported as having macular disease, 15 eyes (5.9%) had glaucoma, six eyes (2.4%) had corneal guttata, five eyes (2.0%) had floppy iris syndrome, two eyes (0.8%) had pseudoexfoliation, and one eye (0.4%) had prior refractive surgery.
For all subjects, the primary incision type was clear cornea, and the mean estimated incision size ± SD was 2.72±0.22 mm. The targeted mean ± SD postoperative manifest refraction spherical equivalent (MRSE) was −0.53±0.67 diopters (D). IOL powers used ranged from +15.00 to +28.50 D. Data acquired at visit 6 (366–730 days postoperative) is reported.
Visual and refractive outcomes
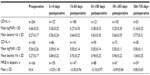
Table 1 shows UDVA and CDVA results in logMAR (logarithm of the minimum angle of resolution) and decimal visual acuity at each interval in addition to MRSE outcomes. Visual outcomes indicated good visual and refractive stability, with gradual improvement over time. A graphical presentation of UDVA results at postoperative visits 4 (61–180 days postoperative) and 5 (181–365 days postoperative) can be found in Figure 3. A graph of the number of eyes achieving a Snellen CDVA of 20/40 (decimal 0.50) or better is presented in Figure 4.
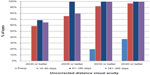  | Figure 3 Graph displaying uncorrected distance visual acuity results preoperatively and at three postoperative visits. |
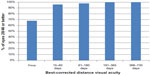  | Figure 4 Graph presenting the percentage of eyes having Snellen best-corrected visual acuity of ≥20/40 (decimal 0.50) preoperatively and at four postoperative visits. |
The change in mean MRSE was 0.01 D between postoperative visits 4 and 5, and 0.09 D between visits 5 and 6, demonstrating refractive stability. Because the target MRSE was not a plano-refraction in all cases, the analysis was performed using typical inclusion criteria for monofocal studies. Eyes included in the accuracy to target analysis had a postoperative MRSE target of ±0.37 D, preoperative keratometric cylinder of ≤0.75 D, and did not undergo additional refractive procedures such as limbal relaxing incisions (five eyes [2.0%]). The accuracy to target MRSE results are presented in Table 2.
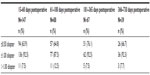  | Table 2 Accuracy to target manifest refraction spherical equivalent |
AEs and secondary interventions
The incidence of AEs experienced during the study was comparable to or lower than the incidence reported in the historical control population per FDA draft guidance.16 All AEs and complications were consistent with typical incidences accompanying cataract or implant surgery. There were no unique reports associated with the MX60 IOL.
No glistenings of any grade were reported for any subject at any visit. One eye (1.0%) received an Nd:YAG capsulotomy at 363 days post-procedure. Three eyes (1.2%) had secondary interventions, which included an intravitreal injection of bevacizumab (Avastin®; Genentech, South San Francisco, CA, USA), suture removal, and a paracentesis to lower the intraocular pressure. No IOL explantations were performed.
Discussion
The results of this study provide evidence of the safety and effectiveness of the MX60 IOL in a real-world clinical setting. At visit 4, 62.2% of the subjects achieved a Snellen CDVA of 20/20, and 97.8% of the subjects achieved a CDVA of 20/40 or better. In addition, the MX60 displayed visual and refractive stability over time. This IOL is designed to maximize the haptic contact angle under compression, preventing ovalization and/or striae of the capsular bag. This is intended to provide refractive stability under compression. In regards to safety, intraoperative and postoperative ocular AEs were of the type and frequency generally observed in patients who have had cataract surgery.17,18 As study outcomes were intended to reflect those of real-life practices, the results of this open-label, nonrandomized study may be subject to selection bias.
PCO is the most common visually disabling sequela of modern cataract surgery,19 caused primarily by the migration of lens epithelial cells that persist after cataract surgery.20 In this study, only one eye (1.0%) underwent Nd:YAG capsulotomy at 12 months post-procedure. In contrast, a retrospective study involving AcrySof IOLs reported an Nd:YAG capsulotomy rate of 17.3% (13 of 75 eyes) for the monofocal spherical group (Natural SN60AT; Alcon Laboratories, Inc.) and 4.0% (3 of 75 eyes) for the monofocal aspheric group (IQ SN60WF; Alcon Laboratories, Inc.); the mean time from surgery to Nd:YAG capsulotomy was 13.0±9.3 months in the monofocal spherical group and 9.3±6.4 months in the monofocal aspheric group.20
Nixon and Woodcock15 reported that IOL design rather than material is the critical factor in minimizing lens epithelial cell migration across the posterior capsule. In their study, characteristics that led to lower levels of PCO with a continuous-edge IOL were the 360° square edge, angled haptics, increased optic–haptic space, and increased resistance to compression. These features help to position the IOL against the posterior capsule and encourage complete circumferential shrink-wrapping of the IOL by the capsule. Apple et al21 surmised that PCO can develop when a potential space is available into which lens epithelial cells can migrate and proliferate. The MX60 features step-vaulted haptics that translate the optic posteriorly for direct contact with the capsular bag. It also has a 360° square barrier edge to inhibit lens epithelial cell migration, thus limiting the development of PCO.
In the study reported here, no glistenings of any grade were reported for any subject at any visit. This observation is corroborated by results from other studies7,22 investigating this same IOL material. Christiansen et al6 reported that the Snellen acuity in eyes with severe glistenings (grade ≥2+) was half a line lower than in eyes with mild glistenings (P=0.01), a finding supported by other studies.23,24 Investigations involving other hydrophobic acrylic IOLs have reported that glistenings appear to increase in severity with time,5,25–27 which was not found with the MX60 IOL. The MX60 IOL is packaged in physiologic saline to eliminate fluid exchange with the aqueous humor. Prehydration of this IOL to equilibrium water content ensures that it remains glistening-free. Following cataract surgery, a pristine IOL is clearly the ideal outcome.
When used to correct aphakia following cataract surgery, the MX60 IOL has demonstrated a well established safety profile with predictable visual outcomes, stable refractive outcomes, a low incidence of Nd:YAG capsulotomies to 12 months post-procedure, and no glistenings.
Acknowledgments
We would like to acknowledge the following participating investigators: Joerg Koch, MD, St Franziskus-Hospital Muenster, Münster, Germany; Michael Kuechle, MD, Augenpraxis-Klinik Erlangen, Erlangen, Germany; Egbert Pool, MD, Ommelander Ziekenhuis Groep, Delfzijl, the Netherlands; Richard Packard, MD, Prince Charles Eye Unit, London, England; Alessandro Franchini, MD, Università Ospedaliera di Firenze Careggi, Firenze, Italy; Javier Mendicute, MD, Hospital de Donostia, San Sebastian, Spain; Manuel Correia, MD, Hospital Narciso Ferreira, Porto, Portugal; Dominique Monnet, MD, PhD, Université Paris Descartes, Paris, France; Pisella Pierre Jean, MD, Hôpital Bretonneau, Tours, France; Andras Berta, MD, University of Debrecen, Debrecen, Hungary; Petr Novak, MD, Hospital Nemocnice Na Homolce, Prague, Czech Republic; Bjorn Johansson, MD, Linköpings Universitetssjukhus, Linköping, Sweden; Gisela Wejde, MD, S:t Eriks ögonsjukhus, Stockholm, Sweden; and Brad Horsburgh, MD, Northside Eye Centre, Nundah, QLD, Australia.
Funding for writing and editorial support was provided by Bausch and Lomb Incorporated, Rochester, NY, USA. Writing and editorial assistance was provided by Stephanie N Baba, OD, Alameda, CA, USA and Gary Mosehauer, MS, Senior Statistician of Bausch and Lomb Incorporated.
Disclosure
This study was sponsored by Bausch and Lomb Incorporated. The authors have no conflicts of interest to disclose.
References
Steinert RF, Puliafito CA, Kumar SR, Dudak SD, Patel S. Cystoid macular edema, retinal detachment, and glaucoma after Nd:YAG laser posterior capsulotomy. Am J Ophthalmol. 1991;112(4):373–380. | |
Rickman-Barger L, Florine CW, Larson RS, Lindstrom RL. Retinal detachment after neodymium:YAG laser posterior capsulotomy. Am J Ophthalmol. 1989(5);107:531–536. | |
Newland TJ, McDermott ML, Eliott D, et al. Experimental neodymium:YAG laser damage to acrylic, poly(methyl methacrylate), and silicone intraocular lens materials. J Cataract Refract Surg. 1999;25(1):72–76. | |
Holweger RR, Marefat B. Intraocular pressure change after neodymium: YAG capsulotomy. J Cataract Refract Surg. 1997;23(1):115–121. | |
Werner L. Glistenings and surface light scattering in intraocular lenses. J Cataract Refract Surg. 2010:36(8);1398–1420. | |
Christiansen G, Durcan EJ, Olson RJ, Christiansen K. Glistenings in the AcrySof intraocular lens: pilot study. J Cataract Refract Surg. 2001;27(5):728–733. | |
Packer M, Fry L, Lavery KT, et al. Safety and effectiveness of a glistening-free single-piece hydrophobic acrylic intraocular lens (enVista™). Clin Ophthalmol. 2013;7:1905–1912. | |
Dixon JR Jr. The International Conference on Harmonisation Good Clinical Practice Guideline. Qual Assur. 1998;6(2):65–74. | |
EnVista HydroStable Acrylic AO Lens [package insert]. Rochester, NY: Bausch and Lomb Incorporated; 2012. | |
Mentak K, Goldberg E, El-Achchabi A. Nanoindentation studies on hydrophobic acrylic iols to evaluate surface mechanical properties. Paper presented at the XXV Congress of the European Society of Cataract and Refractive Surgeons – ESCRS, Sep 2007, Stockholm, Sweden. | |
Eom Y, Lee JS, Rhim JW, et al. A simple method to shorten the unfolding time of pre-hydrated hydrophobic intraocular lens. Presented in part at the Asia-Pacific Association of Cataract and Refractive Surgeons annual meeting in Singapore (July 11–14, 2013) and the 109th meeting of the Korean Ophthalmological Society in South Korea (April 20–21, 2013). | |
Caporossi A, Martone G, Casprini F, Rapisarda L. Prospective randomized study of clinical performance of 3 aspheric and 2 spherical intraocular lenses in 250 eyes. J Refract Surg. 2007;23(7):639–648. | |
Pepose JS, Qazi MA, Edwards KH, Sanderson JP, Sarver EJ. Comparison of contrast sensitivity, depth of field and ocular wavefront aberrations in eyes with an IOL with zero versus positive spherical aberration. Graefes Arch Clin Exp Ophthalmol. 2009;247(7):965–973. | |
Santhiago MR, Netto MV, Barreto J Jr, et al. Wavefront analysis, contrast sensitivity, and depth of focus after cataract surgery with aspherical intraocular lens implantation. Am J Ophthalmol. 2010;149(3):383–389. | |
Nixon DR, Woodcock MG. Pattern of posterior capsule opacification models 2 years postoperatively with 2 single-piece acrylic intraocular lenses. J Cataract Refract Surg. 2010;36(6):929–934. | |
Guidance for Industry and for FDA Reviewers: Intraocular Lens Guidance Document, [Draft Guidance accessed Mar 2013]. | |
Parulekar MV. Complications following cataract surgery. In: Malhotra R, editor. Cataract. Philadelphia: Elsevier Ltd; 2008:141–182. | |
McKellar MJ, Elder MJ. The early complications of cataract surgery: is routine review of patients 1 week after cataract extraction necessary? Ophthalmology. 2001;108(5):930–935. | |
Ram J, Kaushik S, Brar GS, Gupta A. Neodymium:YAG capsulotomy rates following phacoemulsification with implantation of PMMA, silicone, and acrylic intraocular lenses. Ophthalmic Surg Lasers. 2001;32(5):375–382. | |
Biber JM, Sandoval HP, Trivedi RH, Fernández de Castro LE, French JW, Solomon KD. Comparison of the incidence and visual significance of posterior capsule opacification between multifocal spherical, monofocal spherical, and monofocal aspheric intraocular lenses. J Cataract Refract Surg. 2009;35(7):1234–1238. | |
Apple DJ, Peng Q, Visessook N, et al. Eradication of posterior capsule opacification; documentation of a marked decrease in Nd:YAG laser posterior capsulotomy rates noted in an analysis of 5,416 pseudophakic human eyes obtained postmortem. Ophthalmology. 2001;108(3):505–518. | |
Tetz MR, Werner L, Schwahn-Bendig S, Batlle JF. Prospective Clinical Study to Quantify Glistenings in New Hydrophobic Acrylic IOL. Paper presented at the ASCRS Symposium on Cataract, IOL and Refractive Surgery, San Francisco, California, USA, Apr 2009. | |
Dhaliwal DK, Mamalis N, Olson RJ, et al. Visual significance of glistenings seen in the AcrySof intraocular lens. J Cataract Refract Surg. 1996;22(4):452–457. | |
Gunenc U, Oner FH, Tongal S, Ferliel M. Effects on visual function of glistenings and folding marks in AcrySof intraocular lenses. J Cataract Refract Surg. 2001;27(10):1611–1614. | |
Tognetto D, Toto L, Sanguinetti G, Ravalico G. Glistenings in foldable intraocular lenses. J Cataract Refract Surg. 2002;28(7):1211–1216. | |
Behndig A, Monestam E. Quantification of glistenings in intraocular lenses using Scheimpflug photography. J Cataract Refract Surg. 2009;35(1):14–17. | |
Colin J, Praud D, Touboul D, Schweitzer C. Incidence of glistening with the latest generation of yellow-tinted hydrophobic acrylic intraocular lenses. J Cataract Refract Surg. 2012;38(7):1140–1146. | |
Packer M, Fry L, Lavery KT, Lehmann R, et al. Safety and effectiveness of a glistening-free single-piece hydrophobic acrylic intraocular lens (enVista). Clin Ophthalmol. 2013;7:1905–1912. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.