Back to Journals » OncoTargets and Therapy » Volume 8
Relationship between single-nucleotide polymorphism of matrix metalloproteinase-2 gene and colorectal cancer and gastric cancer susceptibility: a meta-analysis
Authors Wu Z, Jiang P, Zulqarnain H, Gao H, Zhang W, Xu X
Received 24 November 2014
Accepted for publication 4 March 2015
Published 16 April 2015 Volume 2015:8 Pages 861—869
DOI https://doi.org/10.2147/OTT.S78031
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Zesheng Wu,1 Peng Jiang,2 Haider Zulqarnain,1 Hua Gao,1 Wenbin Zhang1
1Department of Gastrointestinal Surgery, The First Affiliated Hospital of Xinjiang Medical University, 2Department of Oncology, Tumor Hospital of Xinjiang Medical University, Urumqi, People’s Republic of China
Background: Recently, the published data on the association between matrix metalloproteinase-2 (MMP-2) (C-1306T) polymorphism and colorectal cancer (CRC) and gastric cancer (GC) (gastrointestinal cancer) risk remained controversial. The aim of this study is to investigate the relationship between the risk of CRC and GC and single-nucleotide polymorphism of MMP-2(C-1306T).
Methods: Medline, Embase, Science Citation Index, and PubMed were thoroughly searched to identify relevant studies. Odds ratio (OR) with 95% confidence interval (CI) was used to assess the strength of the association.
Results: We performed a meta-analysis of 14 studies including 642 cases and 692 controls for CRC and 1,936 cases and 3,490 controls for GC. The result indicates that there is significant relationship between MMP-2(C-1306T) polymorphism and CRC risk in recessive model and codominant model (TT vs CC/CT: OR: 2.39, 95% CI: 1.30–4.37, P=0.005; TT vs CC: OR: 2.36, 95% CI: 1.29–4.34, P=0.006). In subgroup analysis according to ethnicity, significant associations were found in Caucasians (TT vs CC/CT: OR: 2.87, 95% CI: 1.43–5.78, P=0.003; TT vs CC: OR: 2.86, 95% CI: 1.41–5.80, P=0.003), but we did not find significant evidence with GC in all genetic models, and in stratified analysis according to ethnicity, no significant risk was found in the subgroup too.
Conclusion: This meta-analysis considered that the MMP-2(C-1306T) polymorphism is a risk factor for CRC susceptibility, especially in Caucasians, but it does not support any relationship to GC, and further studies are needed to explore the association.
Keywords: matrix metalloproteinase-2, colorectal cancer, gastric cancer
Introduction
The incidence of gastrointestinal cancer has increased year by year, with approximately 2 million new cases diagnosed worldwide and approximately 1.2 million patients dying per year. Gastrointestinal cancer ranked in the top five in cancer mortality rankings, the leading cause of cancer death.1,2 Moreover, various environmental factors are major risk factors, especially genetic background.3 Matrix metalloproteinases (MMPs) are a multigene family of zinc-dependent endopeptidases that share a similar structure and collectively have the capacity to degrade essentially all extracellular matrix components.4 Matrix metalloproteinase-2 (MMP-2) is an important member of the family, and the main effect of the MMP-2 is to degrade type IV collagen which is an important part of the cell layer of basement membrane,5 which is involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, angiogenesis,6–8 and tissue remodeling, as well as in disease processes, tumor invasion, and metastasis. A number of researches have demonstrated the role of MMP-2 in colorectal cancer (CRC) and gastric cancer (GC).9–13 Single-nucleotide polymorphism (SNP) is the most common type of genetic variation, and small part of these polymorphisms has function. Most of the functional polymorphisms are located in the promoter region of the gene and are therefore considered to influence gene expression.14–17 Human MMP-2 promoter has been proved to contain several cis-acting regulatory elements. Among them, functional SNP in the promoter region of the MMP-2 (the C-1306T/rs 243865) that disrupts an Sp1-type promoter site (CCACC box) affects MMP-2 expression or activity and may predispose to disease conditions.18 Moreover, transient transfection experiment had shown that MMP-2 expression is ~1.4- to twofold higher with the C allele than with the T allele.19 Many studies have shown that SNP of MMP-2(C-1306T) genes may be associated with gastrointestinal cancer risk. However, as a result of conflicting results from various studies, the relationship between the polymorphisms and gastrointestinal risk remains inconclusive. Hence, we performed a meta-analysis to clarify clinical impact of MMP-2(C-1306T) polymorphism on CRC and GC.
Materials and methods
Search strategy
Medline, Embase, Science Citation Index, and PubMed were thoroughly searched with the terms “metalloproteinases” or “MMPs”, “polymorphism” or “polymorphisms”, “risk”, “susceptibility”, and “colorectal cancer” or “gastric cancer” or “gastrointestinal cancer” (till July 2014). We got more relevant articles by literature references backtracking, and only the publications with full text available were included. Additionally, abstracts and unpublished reports were not considered.
Selections of studies
The following are included in the inclusion criteria: (1) independent case-control design was used to evaluate the association between MMP-2(C-1306T) and the risk of CRC or GC; (2) genotype distribution of the control population that conformed to Hard–Weinberg equilibrium (HWE); (3) the study presented sufficient data to estimate odds ratios (ORs) and 95% confidence intervals (CIs).
The exclusion criteria included the following: (1) there is no comparison, just the study of case or noncancer; (2) literature data that are not complete; (3) the genotype distribution of the control population that did not accord with HWE; (4) if finding overlaps studies, only the most recent or complete study was included in this meta-analysis.
Data extraction
Two investigators read all the included literatures carefully, and then extracted all data such as the first author, published year, ethnicity of study population (Asian or Caucasian), numbers of case and controls, genotype distribution, genotyping methods, and allele independently. If two investigators had divergent idea on any data, another investigator would be asked to check and to reach consensus on the data.
Statistical analysis
HWE was assessed by using the goodness-of-fit χ2 test for control group, and bias was considered when P<0.05. Crude ORs with their corresponding 95% CIs were used to evaluate the strength of relationship between the MMP-2(C-1306T) and the risk of CRC and GC. The pooled ORs were evaluated in codominant model (CT vs CC, TT vs CC), dominant model (CT/TT vs CC), and recessive model (TT vs CC/CT). Subgroup analyses were performed by ethnicity. Moreover, we performed sensitivity analysis to assess the accuracy and stability of the results by excluding a single study each time. Q-test was used to check heterogeneity among the studies. If P<0.1, it is considered as heterogeneity and statistically significant. We used the random-effects model to calculate the pooled OR. Otherwise, the fixed-effects model was used.20,21 Potential publication bias was assessed by Begg’s funnel plot22 and Egger’s test,23 if P<0.05 was considered as statistically significant. Data analysis was performed using Revman 5.1 (Cochrane Collaboration) and Stata 11.0 (Stata Corporation, College Station, TX, USA).
Results
Study characteristics
According to the search strategy, 67 publications were found. Among these studies, 12 articles were irrelevant; 20 were letters, review articles, and meta-analysis; 13 studies were relevant to other members of the MMP family or other polymorphisms of MMP-2; and eight studies were duplicate of a previous study. So, 14 studies were finally included in this meta-analysis (Figure 1).24–37 Fourteen studies which consist of six CRC studies24–29 (642 cases and 692 controls) and eight GC studies30–37 (1,936 cases and 3,490 controls) were included. As illustrated in Table 1, among these studies, nine were Asians and five were Caucasians. In no study was the genotypic distribution of the controls deviated from HWE (P>0.1).
  | Figure 1 Study flow chart explaining the selection of the 14 studies included in the meta-analysis. |
Meta-analysis results
As shown in Table 2, we found that there is significant relationship between MMP-2(C-1306T) polymorphism and CRC risk in recessive model and codominant model (TT vs CC/CT: OR: 2.39, 95% CI: 1.30–4.37, P=0.005; TT vs CC: OR: 2.36, 95% CI: 1.29–4.34, P=0.006; Figures 2 and 3). No significance was found between the other genetic models and CRC. In subgroup analysis according to ethnicity, the results indicated that there is a positive relationship between the MMP-2(C-1306T) polymorphism and CRC risk in Caucasians (TT vs CC/CT: OR: 2.87, 95% CI: 1.43–5.78, P=0.003; TT vs CC: OR: 2.86, 95% CI: 1.41–5.80, P=0.003), but there is no association in Asians. However, when eight GC studies were pooled into the meta-analysis, we found that there is no significant association between MMP-2(C-1306T) polymorphism and GC risk in all genetic models. Therefore, the stratified analysis was performed according to ethnicity; still, no relationship was found between the two subgroups in all genetic models.
Heterogeneity analysis
As shown in Table 3, significant heterogeneity was found between the MMP-2(C-1306T) polymorphism and CRC risk in the dominant model (CT/TT vs CC: χ2=11.83, I2=58%, PH=0.04). To explore the sources of heterogeneity, the stratified analysis was performed according to ethnicity. We found that heterogeneity still exists in the dominant model among Asians (CT/TT vs CC: χ2=6.18, I2=68%, PH=0.05). In order to find further sources of heterogeneity, Galbraith plot analysis was performed to identify which study may result in the heterogeneity. As illustrated in Figure 4, all studies were inside the CI of the regression line. It indicates that overall heterogeneity in dominant model is not significant.38 In addition, there was moderate heterogeneity between MMP-2(C-1306T) polymorphism and GC risk in the codominant model and dominant model (CT vs CC: χ2=33.55, I2=79%, PH<0.05; CT/TT vs CC: χ2=38.97, I2=82%, PH<0.05). Heterogeneity was found among Asians in the same genetic models according to stratified analysis (CT vs CC: χ2=28.9, I2=82%, PH<0.05; CT/TT vs CC: χ2=35.23, I2=86%, PH<0.05). As illustrated in Figures 5 and 6, the studies by Kim et al33 and Miao et al37 were outliers in codominant model and dominant model from the Galbraith plot analysis, and all I2 values decreased obviously, and PH values were more than 0.10 after removing the two studies in all genetic comparison models in the overall populations (CT vs CC: I2=19%, PH=0.29; CT/TT vs CC: I2=17%, PH=0.30), Asians (CT vs CC: I2=0%, PH=0.42; CT/TT vs CC: I2=30%, PH=0.23). The significance of MMP-2(C-1306T) polymorphism in different genetic models in overall population and stratified analysis were not influenced by excluding the two studies.
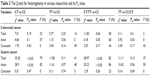  | Table 3 The Q-test for heterogeneity in various researches and its PH value |
  | Figure 4 Galbraith plots of MMP-2(C-1306T) polymorphism and CRC risk in dominant model CT/TT vs CC. |
Sensitivity analysis
As the sample size for case and control in all studies is not the same, which ranged from 50 to 789, we gradually removed the small sample size, and corresponding overall results were not qualitatively altered.
Publication bias
No publication bias could be discovered in any genetic models by Begg’s funnel plot and Egger’s test. All P-values of Egger’s tests were more than 0.05, which present statistical evidence of the funnel plots symmetry (Figure 7 and Table 4).
  | Table 4 The Egger’s test for publication bias in different genetic models and its PE value |
Discussion
MMP was classified as a large family of zinc-containing proteases, and this family is proven to be of relevance for cancer development and prognosis in various systems.39,40 As MMP-2 is an enzyme with proteolytic activity against matrix and non-matrix proteins, particularly basement membrane constituents, and has type IV collagenolytic activity, it is considered to be an important member in the family. It is expressed by great majority of connective tissue cells. Furthermore, researches have also demonstrated that MMPs are associated with the angiogenic switch; it was reliably recapitulated in the model of tumor progression. This switch is the earliest stage that involves in the tumor growth and progression, and MMP-2 was shown to play a key role in the development of the angiogenic phenotype.41 MMP promoter SNPs affecting the gene transcription and expression are associated with malignant cancers susceptibility. SNP is the most common type of genetic variation, and genetic variation in MMP-2 may contribute to matrix membrane damage and angiogenesis, thus increasing cancer risk. The number of SNPs in the human genome is huge, but only a very small part of these polymorphisms have functional properties. Most of the functional polymorphisms are situated in the promoter region of the gene. MMP-2(C-1306T) was deemed to be an important polymorphism in the promoter region of the MMP-2 gene, and it was reported that the polymorphism 1306C→T disrupts an Sp1-type promoter site (CCACC box). Multifunctional protein which can directly interact with the basal transcriptional complex for the MMP-2 proximal promoter may contribute to interindividual diversity in susceptibility to cancer and many complex diseases leading to strikingly lower promoter activity with the T allele.19,42
In this study, the significant relationship between MMP-2(C-1306T) polymorphism and CRC risk was found in recessive model and codominant model (TT vs CC/CT: OR: 2.39, 95% CI: 1.30–4.37, P=0.005; TT vs CC: OR: 2.36, 95% CI: 1.29–4.34, P=0.006), and this positive relationship is more meaningful in European populations after subgroup analysis according to ethnicity (TT vs CC/CT: OR: 2.87; 95% CI: 1.43–5.78, P=0.003; TT vs CC: OR: 2.86, 95% CI: 1.41–5.80, P=0.003). Among the eligible publications, there are three studies that took Europeans as an object of study. Elander et al26 did not find any evidence for the associations regarding CRC and MMP-2(C-1306T) polymorphism. However, this was different from Saeed et al’s and Hesham et al’s studies;27,28 they all found that MMP-2(C-1306T) polymorphism might increase the risk of CRC. Furthermore, Hesham also found that both the homozygous TT and T alleles were significantly associated with higher risk of colorectal cancerogenesis in males and old-aged patients. But both Saeed’s and Hesham’s researches took Saudi population as an object of study, so further investigations are needed to confirm and clarify whether this observation is only due to different regions. In Asians, no significant relationship was found between the risk of developing CRC and MMP-2(C-1306T) polymorphism from Kang’s and Ohtani’s studies.24,29 In contrast to this, Xu’s study25 suggested that MMP-2(C-1306T) polymorphism may be associated with CRC development, and found that CRCs with CC genotype were more common with serosa/adventitia layer invasiveness compared with other genotypes (OR: 1.959, 95% CI: 1.055–3.637).
However, when eight GC studies were pooled into the meta-analysis, we found that there is no significant association between MMP-2(C-1306T) polymorphism and GC risk in all genetic models; the same result appeared in the stratified analysis. Our results were consistent with those reports from Wu et al’s, Kubben et al’s, Li et al’s, Kim et al’s, Alakus et al’s, and Lin et al’s studies;30–34,36 they all did not find significant difference in distribution of MMP-2(C-1306T) polymorphism between GC patients and controls. In addition, in contrast to Alakus’s study,34 Kubben’s study31 showed that tumor of patients with the CC genotype contained significantly more MMP-2 antigen than tumor of patients with the CT/TT genotypes. The different methods used to determine antigen levels of MMP-2 in the different studies may contribute to these different results.43 Further investigations are needed to clarify these different findings. Nevertheless, Zhang et al’s and Miao et al’s studies35,37 found that subjects with the CC genotype had increased risk of developing GC compared with other genotypes. Finally, we exclude Liu et al’s study to include 344 GC patients and 324 controls because only the data of TT/CT genotype and CC genotype were presented in the study. Liu et al suggested that MMP-2(C-1306T) polymorphism is an important risk factor for GC and the multifactor interactions (OR: 3.07, 95% CI: 2.09–4.50).44 Therefore, if we include their study, our conclusion may be changed.
We also found six recent meta-analyses focused on the gastrointestinal cancer risk and MMP-2(C-1306T) polymorphism.10,45–49 Only Langers et al’s study reported that the association between MMP-2(C-1306T) polymorphism and gastrointestinal cancer risk is not unidirectional (OR: 2.77, 95% CI: 1.27–6.04).49 The remaining studies did not find an association between the polymorphism and CRC, but those meta-analyses did not include two studies that reported that the MMP-2(C-1306T) genotype was associated with a significant increase in CRC susceptibility. In the Saeed’s and Hesham’s study27,28 cohort of 220 CRC patients and 241 control patients, samples of these different areas may significantly affect the results. Moreover, Peter et al’s study48 includes one duplicate of a previously published study, which may affect the accuracy of the conclusion. Yang et al’s study found that there was no association between the risk of GC and this polymorphism in dominant and recessive models;46 none of the other studies found similar results.10,45,49 Li’s meta-analyses included the study that suggested that MMP-2(C-1306T) polymorphism is an important risk factor for GC and the multifactor interactions (OR: 0.68, 95% CI: 0.47–0.99).44 Moreover, Peng’s result of meta-analysis showed that MMP-2(C-1306T) TT and TC genotype carriers were less susceptible to GC compared with CC genotype carrier. The meta-analyses of Peng and Langers included four studies, a relatively small number for studying the influence of gene polymorphisms on cancer susceptibility. This may explain the discordant results between the different studies and illustrates the need for larger sample sizes.
This meta-analysis might have several limitations. First, the controls were not uniformly defined. Most of them were common hospital-based case-control studies; other controls were population based. Hence, selection bias cannot be fully excluded. It would therefore be important to confirm these findings in a population-based prospective study. Second, our study has high heterogeneity in some genetic models; this may have insufficient statistical power to check the association. In addition, because of data limitations, this study is unable to adjust other environmental risk factors such as age, alcohol consumption, and pathogenic infections.
In summary, this meta-analysis indicates that the MMP-2(C-1306T) polymorphism is a risk factor for CRC susceptibility, especially in Caucasians, but it does not support any relationship to GC. However, because of data limitations, this study may not be particularly perfect. Therefore, further large sample studies are needed to estimate the effect of gene–gene and gene–environment interactions, and studies including more samples with different ethnicities, environmental factors, and sufficient biological evidence for the SNP functions may lead to a better, comprehensive understanding of the association between the MMP-2(C-1306T) polymorphism and gastrointestinal cancer risk.
Acknowledgment
This research was supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region (2014211C036). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Lorenzen S, Sporl S, Lordick F. [Incidence and treatment of chemotherapy-induced nausea and emesis in gastrointestinal cancer]. Z Gastroenterol. 2014;52(8):821–830. | ||
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Companioni O, Bonet C, Muñoz X, et al. Polymorphisms of Helicobacter pylori signaling pathway genes and gastric cancer risk in the European prospective investigation into cancer-eurgast cohort. Int J Cancer. 2014;134(1):92–101. | ||
Surlin V, Ioana M, Plesea IE. Genetic patterns of metalloproteinases and their tissular inhibitors – clinicopathologic and prognostic significance in colorectal cancer. Rom J Morphol Embryol. 2011;52(1 suppl):231–236. | ||
Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol. 2003;36(1):128–137. | ||
Giannopoulos G, Pavlakis K, Parasi A, et al. The expression of matrix metalloproteinases-2 and -9 and their tissue inhibitor 2 in pancreatic ductal and ampullary carcinoma and their relation to angiogenesis and clinicopathological parameters. Anticancer Res. 2008;28(3B):1875–1881. | ||
Chetty C, Lakka SS, Bhoopathi P, Rao JS. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer. 2010;127(5):1081–1095. | ||
Foda HD, Zucker S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov Today. 2001;6(9):478–482. | ||
Murnane MJ, Cai J, Shuja S, McAneny D, Klepeis V, Willett JB. Active MMP-2 effectively identifies the presence of colorectal cancer. Int J Cancer. 2009;125(12):2893–2902. | ||
Peng B, Cao L, Ma X, Wang W, Wang D, Yu L. Meta-analysis of association between matrix metalloproteinases 2, 7 and 9 promoter polymorphisms and cancer risk. Mutagenesis. 2010;25(4):371–379. | ||
Wang K, Sun XJ, Li SG, Shang WH, Jia PB, Feng HA. Expressions of matrix metalloproteinase 2 and carbohydrate antigen 50 in colorectal carcinoma, transitional mucosa and normal colorectal mucosa and its clinical significance. Chin J Bases Clin Gen Sure. 2006;13:417–420. | ||
Jeffery N, McLean MH, El-Omar EM, Murray GI. The matrix metalloproteinase/tissue inhibitor of matrix metalloproteinase profile in colorectal polyp cancers. Histopathology. 2009;54(7):820–828. | ||
Feng G, Tan Y. [Expression and significance of MMP2 and type IV collagen in gastric cancer]. Zhonghua Wai Ke Za Zhi. 2000;38(10):775–777. | ||
Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253(1–2):269–285. | ||
Yu C, Zhou Y, Miao X, Xiong P, Tan W, Lin D. Functional haplotypes in the promoter of matrix metalloproteinase-2 predict risk of the occurrence and metastasis of esophageal cancer. Cancer Res. 2004;64(20):7622–7628. | ||
Vasků A, Goldbergová M, Izakovicová Hollá L, et al. A haplotype constituted of four MMP-2 promoter polymorphisms (-1575G/A, -1306C/T, -790T/G and -735C/T) is associated with coronary triple-vessel disease. Matrix Biol. 2004;22(7):585–591. | ||
Harendza S, Lovett DH, Panzer U, Lukacs Z, Kuhnl P, Stahl RA. Linked common polymorphisms in the gelatinase a promoter are associated with diminished transcriptional response to estrogen and genetic fitness. J Biol Chem. 2003;278(23):20490–20499. | ||
Gonçalves FM, Martins-Oliveira A, Lacchini R, et al. Matrix metalloproteinase (MMP)-2 gene polymorphisms affect circulating MMP-2 levels in patients with migraine with aura. Gene. 2013;512(1):35–40. | ||
Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation. J Biol Chem. 2001;276(10):7549–7558. | ||
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. | ||
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. | ||
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. | ||
Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Kang MJ, Jung SA, Jung JM, et al. Associations between single nucleotide polymorphisms of MMP2, VEGF, and HIF1A genes and the risk of developing colorectal cancer. Anticancer Res. 2011;31(2):575–584. | ||
Xu E, Lai M, Lv B, Xing X, Huang Q, Xia X. A single nucleotide polymorphism in the matrix metalloproteinase-2 promoter is associated with colorectal cancer. Biochem Biophys Res Commun. 2004;324(3):999–1003. | ||
Elander N, Soderkvist P, Fransen K. Matrix metalloproteinase (MMP) -1, -2, -3 and -9 promoter polymorphisms in colorectal cancer. Anticancer Res. 2006;26(1B):791–795. | ||
Saeed HM, Alanazi MS, Parine NR, et al. Matrix metalloproteinase-2 (-1306 c>t) promoter polymorphism and risk of colorectal cancer in the Saudi population. Asian Pac J Cancer Prev. 2013;14(10):6025–6030. | ||
Hesham MS, Nounou HA, Alanazi MS, Alharby O, Azzam N, Saeed HM. Associations between single nucleotide polymorphisms of COX-2 and MMP-2 genes and colorectal cancer susceptibility in the Saudi population. Asian Pac J Cancer Prev. 2014;15(12):4989–4994. | ||
Ohtani H, Maeda N, Murawaki Y. Functional polymorphisms in the promoter regions of matrix metalloproteinase-2, -3, -7, -9 and TNF-alpha genes, and the risk of colorectal neoplasm in Japanese. Yonago Acta Med. 2009;52(1):47–56. | ||
Wu CY, Wu MS, Chen YJ, et al. Clinicopathological significance of MMP-2 and TIMP-2 genotypes in gastric cancer. Eur J Cancer. 2007;43(4):799–808. | ||
Kubben FJ, Sier CF, Meijer MJ, et al. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. Br J Cancer. 2006;95(6):744–751. | ||
Li Y, Sun DL, Duan YN, et al. Association of functional polymorphisms in MMPs genes with gastric cardia adenocarcinoma and esophageal squamous cell carcinoma in high incidence region of North China. Mol Biol Rep. 2010;37(1):197–205. | ||
Kim J, Pyun JA, Cho SW, Lee K, Kwack K. Lymph node metastasis of gastric cancer is associated with the interaction between poly (ADP-ribose) polymerase 1 and matrix metallopeptidase 2. DNA Cell Biol. 2011;30(12):1011–1017. | ||
Alakus H, Afriani N, Warnecke-Eberz U, et al. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. World J Surg. 2010;34(12):2853–2859. | ||
Zhang XM, Miao XP, Xiong P, et al. [Association of functional polymorphisms in matrix metalloproteinase-2 (MMP-2) and MMP-9 genes with risk of gastric cancer in a Chinese population]. Ai Zheng. 2004;23(11):1233–1237. | ||
Lin XD, Chen G, Li C, et al. [Association of polymorphism in matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 with genetic susceptibility of gastric cancer]. Zhonghua Yu Fang Yi Xue Za Zhi. 2011;45(8):711–716. | ||
Miao X, Yu C, Tan W, et al. A functional polymorphism in the matrix metalloproteinase-2 gene promoter (-1306C/T) is associated with risk of development but not metastasis of gastric cardia adenocarcinoma. Cancer Res. 2003;63(14):3987–3990. | ||
Martin Bland. Meta-Analysis: Dealing with Heterogeneity. Vol. 23. York: University of York; 2006:6. | ||
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. | ||
Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8(12):929–941. | ||
Fang J, Shing Y, Wiederschain D, et al. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci U S A. 2000;97(8):3884–3889. | ||
Grieu F, Li WQ, Iacopetta B. Genetic polymorphisms in the MMP-2 and MMP-9 genes and breast cancer phenotype. Breast Cancer Res Treat. 2004;88(3):197–204. | ||
Alakus H, Grass G, Hennecken JK, et al. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23(8):917–923. | ||
Liu L, Wu C, Wang Y, et al. Association of candidate genetic variations with gastric cardia adenocarcinoma in Chinese population: a multiple interaction analysis. Carcinogenesis. 2011;32(3):336–342. | ||
Li X, Qu L, Zhong Y, Zhao Y, Chen H, Daru L. Association between promoters polymorphisms of matrix metalloproteinases and risk of digestive cancers: a meta-analysis. J Cancer Res Clin Oncol. 2013;139(9):1433–1447. | ||
Yang TF, Guo L, Wang Q. Meta-analysis of associations between four polymorphisms in the matrix metalloproteinases gene and gastric cancer risk. Asian Pac J Cancer Prev. 2014;15(3):1263–1267. | ||
Liu D, Duan W, Guo H, Xu X, Bai Y. Meta-analysis of associations between polymorphisms in the promoter regions of matrix metalloproteinases and the risk of colorectal cancer. Int J Colorectal Dis. 2011;26(9):1099–1105. | ||
Peter MC, Sharma P. Polymorphisms of matrix metalloproteinases 1, 2, 3 and 9 and susceptibility to lung, breast and colorectal cancer in over 30,000 subjects. Int J Cancer. 2009;125(6):1473–1478. | ||
Langers AM, Verspaget HW, Hommes DW, Sier CF. Single-nucleotide polymorphisms of matrix metalloproteinases and their inhibitors in gastrointestinal cancer. World J Gastrointest Oncol. 2011;3(6):79–98. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.