Back to Journals » ClinicoEconomics and Outcomes Research » Volume 7
Relationship between patient dependence and direct medical-, social-, indirect-, and informal-care costs in Spain
Received 16 January 2015
Accepted for publication 28 April 2015
Published 2 July 2015 Volume 2015:7 Pages 387—395
DOI https://doi.org/10.2147/CEOR.S81045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio L Colombo
Josep Darbà,1 Lisette Kaskens2
1Department of Economics, University of Barcelona, 2BCN Health Economics and Outcomes Research SL, Barcelona, Spain
Objective: The objectives of this analysis were to examine how patients' dependence on others relates to costs of care and explore the incremental effects of patient dependence measured by the Dependence Scale on costs for patients with Alzheimer's disease (AD) in Spain.
Methods: The Co-Dependence in Alzheimer's Disease study is an 18 multicenter, cross-sectional, observational study among patients with AD according to the clinical dementia rating score and their caregivers in Spain. This study also gathered data on resource utilization for medical care, social care, caregiver productivity losses, and informal caregiver time reported in the Resource Utilization in Dementia Lite instrument and a complementary questionnaire. The data of 343 patients and their caregivers were collected through the completion of a clinical report form during one visit/assessment at an outpatient center or hospital, where all instruments were administered. The data collected (in addition to clinical measures) also included sociodemographic data concerning the patients and their caregivers. Cost analysis was based on resource use for medical care, social care, caregiver productivity losses, and informal caregiver time reported in the Resource Utilization in Dementia Lite instrument and a complementary questionnaire. Resource unit costs were applied to value direct medical-, social-, and indirect-care costs. A replacement cost method was used to value informal care. Patient dependence on others was measured using the Dependence Scale, and the Cumulative Index Rating Scale was administered to the patient to assess multi-morbidity. Multivariate regression analysis was used to model the effects of dependence and other sociodemographic and clinical variables on cost of care.
Results: The mean (standard deviation) costs per patient over 6 months for direct medical-, social-, indirect-, and informal-care costs were estimated at €1,028.10 (€1,655.00), €843.80 (€2,684.80), €464.20 (€1,639.00), and €33,232.20 (€30,898.90), respectively. Dependence was independently and significantly associated with direct medical-, social-, informal-, and total-care costs.
Conclusion: The costs of care for patients with AD in Spain are substantial, with informal care accounting for the greatest part. Interventions that reduce patient dependence on caregivers may be associated with important reduction in direct medical-, social-, informal-, and total-care costs.
Keywords: Alzheimer, Dependence Scale, direct medical care costs, social care costs, indirect care costs, informal care costs.
Introduction
Alzheimer’s disease (AD), the most common cause of dementia, is a major cause of disability and care burden in the elderly.1,2 Over time, patients invariably develop cognitive and functional decline, and most develop behavioral disturbances sooner or later.3
AD has been shown to cause a substantial burden on several levels including personal, emotional, financial, and social levels.4 In most countries, a substantial proportion of the total care costs of AD is absorbed by family members caring for patients at home.5 Declining cognitive and functional abilities contributes increasingly to the patient’s loss of independent living and dependency.2,3
The relationship between a patients’ loss of function and a higher cost of care for patients with AD has been clearly demonstrated.2,6–9 To provide a full explanation of variation in AD-related costs and patients’ dependence on other individuals, the Dependence Scale (DS)10 was developed to directly measure the amounts of help AD patients require. Several studies have begun to examine the effect of dependence on costs of care.11–15 Although the effect of dependence is less well known, these studies provide support for the DS as an independent predictor of cost and suggest a positive relationship between increasing dependence and higher costs. Zhu et al8,9 found that dependence measured by the DS has an incremental effect on costs of care for patients with AD above the effects of function. This makes it very important to explore the effect of dependence and functional disability in dementia separately, and the effect of dependence on different cost components of care.
In this study, data on resource utilization for medical care, social care, caregiver productivity losses, and informal caregiver time reported in the Resource Utilization in Dementia Lite instrument and a complementary questionnaire were collected for a sample of patients with AD in Spain over a period of 6 months. The objectives of this study were to examine how patients’ dependence on others relates to costs of care and to estimate the incremental effect of dependence on costs for patients with AD. By estimating these relationships, we hope to provide useful information for future economic evaluations and provide insights for decision-makers for the management of AD, patient support, and health care planning of resources.
Methods
Study sample
The Codep-AD study conducted in 2011–2012 was an 18 multicenter, cross-sectional, observational study among patients with AD according to the Clinical Dementia Rating (CDR) score and their caregivers in Spain. The data of 343 patients and their caregivers were collected through the completion of a clinical report form during a one visit/assessment at an outpatient center or hospital, where all instruments were administered.
Participants for the study for each of the 18 centers were identified at each individual center or hospital. Inclusion criteria required patients to have received a diagnosis of possible or probable AD according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition.16 Probable or possible AD was diagnosed according to the NINCDS-ADRDA criteria.17 The severity of dementia for each patient was established by the global CDR scale score.18 Other criteria included the presence of a reliable and trustworthy caregiver to accompany the patient during the study visit and the person responsible for helping the patient in their basic and instrumental needs of daily life and to provide supervision at home for a minimum of at least 10 hours per week. The caregiver need not to be a member of the family or live with the patient. All patients and responsible caregivers had to sign an informed consent form.
Patients were excluded from the study if they had comorbid illness, which was a significant independent cause of disability (eg, dense hemiplegia or Parkinson’s disease), had a clinical status that predicted an outcome of short-term mortality, if the clinical study investigator opined that the patient and caregiver were not able to comply with the study protocol or if patients were participating in a clinical trial. Local ethics approval was obtained for the study.
A range of data were collected for each participant via a case report form including medical assessments and structured questionnaires. All medical assessments were completed by a physician and a psychologist at the patient’s outpatient center or hospital. All remaining data on sociodemographic and other clinical details, health and social care utilization and caregiving hours, were collected via structured caregiver questionnaires. Summary statistics for sociodemographic characteristics and clinical related variables of the 343 study participants are presented in Table 1.
Dependent cost variables
Five cost variables were identified, estimated, and examined in the statistical analysis. These included 1) medical care costs, 2) social care costs, 3) indirect care costs, 4) informal care costs, and 5) total care costs including all cost variables. Data on resources for the estimation of the five costs variables were obtained from the Resource Utilization in Dementia19 Lite instrument and a complementary questionnaire which included aspects related to the utilization of resources not collected in the Resource Utilization in Dementia Lite questionnaire, both completed by the psychologist of the caregiver. This supplemental questionnaire included, among other aspects, modifications at home to improve the patients’ safety, transport costs of the patient, and pocket money for patient care.
Medical care costs were estimated for a set of resources including hospitalizations, emergency visits, diagnostic and monitoring tests, outpatient specialist visits, health and social care professional consultations, and health materials. Data on utilization over a 6-month period were collected and the total costs for medical care were estimated by applying a unit cost for each resource activity. Unit costs were derived from different local Spanish sources and expressed in € 2013. Prices were updated according to the consumer index by the Spanish National Institute for Statistics (Table 2).20
Social care costs were calculated from estimates of the number of nights living in an institutionalized setting, attendance of a day care center, number of complimentary services (day care at home, help at home, nurse home visits, meal delivery, transport services to day care center), and performed home modifications. Data on utilization were collected and the total costs for social care were estimated by applying a unit cost expressed in € 2013 (Table 2) for each resource activity in number of nights/days, received payments for home modifications, and number of services over the last 6 months.
Indirect care costs associated with lost productivity of the caregiver were calculated from estimates of reduced working hours per month and the loss of full and half working days per month. Unit costs for the loss of productivity were based on the national average wage per hour for a woman and man (% women/men 86.2%) of €11.98 obtained from Spanish National Institute for Statistics expressed in € 2013. The total care costs were estimated by applying the hourly average wage to the lost working hours over a 6-month period, whereas a half lost working day counted for 4 hours and a full working day for 8 hours.20
Informal care costs were calculated from estimates of caregiving hours provided by the primary and secondary caregivers for each patient. This includes the total number of hours dedicated to basic activities of daily living and instrumental activities of daily living over the previous month as well as supervision of the patient. The hours of care per task were summed to obtain an estimate of the daily caregiving hours per patient. As it is difficult to value informal care, a replacement cost approach21 was used to value and quantify the cost of informal caregiver time, whereby all care hours are costed at the level of remuneration required to hire an equivalent professional. For the replacement cost, the hourly rate for health care assistance at home of €15.71 per hour (€ 2013) was used. No distinction was made between employed and unemployed caregivers. The daily informal care cost per patient was calculated by multiplying total care hours by the hourly wage rate and extrapolated to obtain an estimate of informal care cost over a 6-month period. The total care costs including all cost variables were equal to the sum of all costs over a 6-month period. In case extreme values for some direct medical care costs were observed and in case misinterpretation of the type and number of resources was suspected, these resources were excluded to prevent overestimation of costs.
Independent variables
The independent variables adopted in this analysis included a range of sociodemographic characteristics and clinical measures. Sociodemographic data included the patients’ age, sex, years since diagnosis, and place of residence (institutionalized vs carer).
The DS10 measured the amount of assistance patients with dementia required, due to impairments. The questionnaire was completed as a caregiver interview, consisting of 13 questions. The scores of the scale range between 0 and 15 and the scale is scored as a sum of items, with a higher score indicating more impairment and a greater dependence of the patient on a caregiver.
The Cumulative Illness Rating Scale (CIRS)22 was administered to the patient to assess multi-morbidity. The scale consists of 14 dimensions that allow the quantification of chronic conditions considering severity. The scale is scored as the sum for each dimension and although the score ranges between 0 and 56, very high scores are not plausible as they represent concurrent failure of multiple systems which are not compatible with life.
Statistical analysis
Descriptive statistics for the estimated costs of care are presented using univariate analysis of variance (ANOVA) tables in terms of the mean and standard deviation (SD) for the whole sample and stratified by categories of dependence in quartiles (DS: 0–6; DS: 7–8; DS: 9–10; DS: 11–15), with the first quartile representing the lowest level of dependence and the highest quartile the most severe level of dependence.
Multivariate generalized linear regression analyses were carried out to explore the effects of the independent variables on each of the five dependent cost variables. For comparative purposes upon request, the results from a range of alternative model specifications are available from the authors. In each case, the regression model included the following independent variables: DS score, years since diagnosis, CIRS score, patients’ sex (0=Male; 1=Female), living in an institutionalized setting (0=No; 1=Yes), living with the caregiver (0=No; 1=Yes), and the patients’ age.
In all analyses, the dependent cost variable was modeled in its untransformed scale. The regression coefficients for continuous independent variables showed estimates for the unit change in cost for a unit change in that variable. That is, for a unit increase in the explanatory variable, cost increases by 100 beta%. For dichotomous variables, the coefficient estimated the unit change in cost relative to the reference group for that variable. Statistical significance was explored for two levels at P<0.01 and P<0.05. The model comparison was based on log likelihood or cube root statistics. Data were analyzed with SPSS® version 18.0 for Windows (SPSS; Chicago, IL, USA).
Results
The results of the descriptive statistics for medical and non-medical resource utilization, lost caregiver working hours, and informal caregiving hours per day are presented in Table 3.
When all individual medical resources were summed and costed, the mean (SD) direct medical care costs per patient over 6 months were equivalent to €1,028.12 (€1,655.02) (Table 4). With respect to social care costs, indirect care costs due to productivity loss of the primary or/and secondary caregiver and informal care costs, the mean (SD) costs per patient for these cost variables over 6 months were estimated to be €843.80 (€2,684.80), €464.20 (€1,639.00), and €33,232.20 (€30,898.90), respectively. Informal care costs showed to be the highest compared to the other cost variables. When all cost variables were summed, the total overall mean (SD) cost per patient summed up to €32,177.30 (€31,836.90) over 6 months.
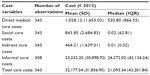  | Table 4 Summary of costs over previous 6 months |
The average 6-month direct medical care costs, social care costs, indirect care costs, and informal care costs by DS score quartiles are presented in Table 5 and show that costs rise with greater levels of dependence. Statistic differences according to DS score quartiles were observed for direct medical-, social-, informal-, and total-care costs (P<0.001). No statistical differences were observed in indirect care costs according to DS score quartiles.
The results from the multivariate analyses are presented in Table 6. The results for direct medical-, social-, and informal-care costs showed that the independent variable which was statistically significant after controlling for other covariates with all these four dependent cost variables was the DS score. Specifically, each additional one-point increase in the DS score, that is a one-unit improvement in patients’ dependence, was associated with a 13.5% increase in direct medical care costs, a 25.3% increase in social care costs, and a 214.7% increase in informal care costs over 6 months significance (P=0.01). No other independent variable was statistically significant for four cost variables.
Regarding direct medical care costs, three independent variables reached statistical significance (P=0.01) in the regression analysis: DS score, years since diagnosis, and CIRS. A one-point increase in years since diagnosis was associated with a 15% (P=0.01) decrease in direct medical care costs over 6 months. A one-point increase in CIRS score was associated with a 6.4% (P=0.01) increase in direct medical care costs over 6 months.
In respect to indirect care costs, the only independent variable which was statistically significant after controlling for other covariates was living with the caregiver. Living with the caregiver was associated with a 101.3% (P=0.05) decrease in indirect medical care costs over 6 months.
Regarding informal care costs, the independent variables which were statistically significant after controlling for other covariates were the DS score and living with the caregiver. Living with the caregiver was associated with a 270.2% (P=0.05) increase in informal care costs over 6 months. No other independent variable reached statistical significance associated with direct medical-, social-, indirect-, and informal-care costs.
In the summed total care costs analysis, both DS scores and sex were significantly associated with total care costs over 6 months. A one-point increase in the DS score was associated with a 185.6% (P=0.01) increase in total care costs, whereas being a woman lead to a 263.5% increase in total care costs over 6 months.
Comparing these results with those from alternative model specifications available upon request to the authors suggests that estimates for the effects of dependence on costs were consistent as the findings remained consistent across alternative model specifications. The observed correlations give an indication of the variables that will be the most influencing and explanatory in the multivariate analysis. Regarding dependence, the only cost variable that is not significantly correlated to quantitative clinical variables is the indirect care costs (supporting information). Multi-morbidity measured by CIRS was significant with all cost variables except with direct non-medical care costs.
Discussion
This analysis presented the estimated resource utilization and costs of direct medical care, social care, productivity loss of caregivers, and informal care for a sample of patients with AD living in the community in Spain. The mean (SD) costs per patient over 6 months for direct medical-, social-, indirect-, and informal-care costs were estimated to be €1,028.10 (€1,655.00), €843.80 (€2,684.80), €464.20 (€1,639.00), and €33,232.20 (€30,898.90), respectively. Total combined mean (SD) costs per patient summed up to €32,177.30 (€31,836.90) over 6 months. The incremental effect of patient dependence on the five total cost care variables was also estimated, while controlling for other clinical measures and a range of other sociodemographic characteristics.
In general, we find that the cost results for the Spanish sample reflect those from recent studies12–15 conducted in different countries except from Spain with informal care being the most important component of costs of care. The sum of the total care costs also showed to increase in the current study as patient DS increases and similar results were observed in the study by Gillespie et al12 and Knapp et al.15
In our study, we also found that patient dependence was associated with direct medical-, social-, informal-, and total-care costs. An increase in dependence was associated with an increase in these costs. Similar results were obtained by various studies8,23,24 showing that patients’ dependence provides an important contribution in explaining variations in health care cost in AD and those changes in dependence are associated with changes in costs of care. This confirms that dependence plays an independent role in explaining variations in costs of care. Our results suggest that small changes in patient dependence may be associated with significant differences in direct medical-, social-, informal-, and total-care costs. One-point increase in the DS score, that is a one-unit improvement in patients’ dependence, was associated with a 13.5% increase in direct medical care costs, a 25.3% increase in social care costs, and a 214.7% increase in informal care costs over 6 months significance (P=0.01). We found that the cost increases as patient DS increases were primarily driven by increases in informal care as was also observed in the study by Gillespie et al;12 though in the study by Knapp et al,15 cost were primarily driven by increases in direct non-medical care costs.
Thus, interventions that enhance patient independence or delay patients’ dependence may be associated with cost savings as well as clinical benefits. However, it is important to evaluate the cost effectiveness of these interventions before introducing and implementing them in clinical practice. For the management of AD, patient support, and health care planning of resources, it is important for decision-makers to focus on these interventions.
Although the focus of the paper was to explore the impacts of dependence on cost, we also identified other significant effects of CIRS, years since diagnosis, sex, and living with the carer on some of the different cost components. This may indicate that except dependence some of these other independent covariates have an effect on costs and are also drivers of some of the different cost components. As no data were collected on the socio-economic status of the patients, any relationship with patient dependence and its effect on costs could not be explored.
The strength of this study includes the use of a structured assessment procedure in which numerous validated instruments are applied as well as the collection of a broad range of resources for each participant including direct medical care, social care, productivity loss of caregivers, and informal caregiver hours. The design of the study allowed us to carry out a comprehensive cost analysis.
Besides, this study also has several limitations that need to be considered when interpreting the results. First, participants with mild, moderate, and severe AD in our study sample were selected from different hospitals in various Spanish regions, and may represent a non-random sample of AD patients in the community. However, because patients were drawn from multiple locations, generalizability of our findings is enhanced. Second, data on patients’ health care costs were reported by patients and caregivers of the patient. In several studies,25,26 it was shown that caregivers are able to accurately report medical information of the patients they take care of. Although there is no reason to believe that patients or caregivers’ reports of patients’ health care utilization are inaccurate, differences in the interpretation on the type and number of resources could have influenced the cost outcomes. Here, it must be observed that extreme values for some direct medical care costs have been observed and excluded in case misinterpretation of the type and number of resources was suspected. It is also possible that there are additional costs beyond those collected in the study, which might not have been included. As reported in other studies,8,9 the existence of uncertainty in valuing informal care has been reported, which makes it complicated and controversial.27,28 Normally, informal caregiver time is not reimbursed or available in the market,17 which makes the valuation of caregiver time and results sensitive to the approach adopted. In our analysis, caregiver time was valued including the costs of active care tasks (ie, basic and instrumental activities of daily living) as well as supervision. For both resources, a replacement cost per hour to hire a professional health care assistant was used. Including supervision in the costing of informal care could have increased the contribution of informal care costs to the total and further increase its relative importance to other resources, as reported by Wimo et al.5 Another uncertainty in the assessment of informal care could be the overstatements by some caregivers. Therefore, another limitation is that there may be some costs that might have been counted double since some caregivers may have decreased their hours of work in order to provide informal care giving. It is difficult to quantify the extension and therefore the effect on the reported outcomes, though it should be acknowledged as a limitation.
Finally, the process of the costing of resource activities was complicated by the lack of one data source for all unit cost data. All unit costs are best estimates of the cost per activity. Therefore, it was not possible to identify cost differences across different sites. Further investigation is necessary to examine whether variations in resource utilization and costs reflect regional differences or availability or access of services.
Conclusion
The findings from this study show that levels of dependence for patients with AD were significantly associated with various components of the cost of care. We find that patient dependence is an important predictor of direct medical-, social-, informal-, and total-care costs. Consequently, interventions that enhance patient independence or delay patients’ dependence may be associated with cost savings in direct medical care, social care, and informal care. For the management of AD, patient support, and health care planning of resources, it is important for decision-makers to focus on these interventions.
Acknowledgments
The authors would like to thank all the health care professionals of the study centers and study participants in the project.
Author contributions
Josep Darbà’s and Lisette Kaskens’s contributions include the designing of the study, conducting the statistical analysis and interpretation of the data, and writing and revising the manuscript. Josep Darbà is guarantor of the manuscript.
Disclosure
This study was sponsored by Pfizer Inc and Janssen Alzheimer Immunotherapy Research and Development, LLC. Josep Darbà is employed by the University of Barcelona and was involved as an external advisor hired by Pfizer Inc and Janssen Alzheimer Immunotherapy Research and Development. Lisette Kaskens is an employee of BCN Health, who was paid consultants to Janssen Alzheimer Immunotherapy Research and Development, LLC and Pfizer Inc.
References
Perkins P, Annegers JF, Doody RS, Cooke N, Aday L, Vernon SW. Incidence and prevalence of dementia in a multiethnic cohort of municipal retirees. Neurology. 1997;49:44–50. | |
Jönsson L, Eriksdotter Jönhagen M, Kilander L, et al. Determinants of costs of care for patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21(5):449–459. | |
DeKosky ST, Orgogozo JM. Alzheimer disease: diagnosis, costs, and dimensions of treatment. Alzheimer Dis Assoc Disord. 2001;15(Suppl 1):S3–S7. | |
Reese JP, Hessmann P, Seeberg G, et al. Cost and care of patients with Alzheimer’s disease: clinical predictors in German health care settings. J Alzheimers Dis. 2001;27(4):723–736. | |
Wimo A, Wetterholm AL, Mastey V, Winblad B. Evaluation of the health care resource utilization and caregiver time in anti-dementia drug trials – a quantitative battery. In: Wimo A, Karlsson G, Winblad B, editors. Health Economics of Dementia. Chichester: John Wiley & Sons;1998:465–493. | |
Taylor DH Jr, Schenkman M, Zhou J, Sloan FA. The relative effect of Alzheimer’s disease and related dementias, disability, and comorbidities on cost of care for elderly persons. J Gerontol B Psychol Sci Soc Sci. 2001;56:S285–S293. | |
Small GW, McDonnell DD, Brooks RL, Papadopoulos G. The impact of symptom severity on the cost of Alzheimer’s disease. J Am Geriatr Soc. 2002;50:321–327. | |
Zhu CW, Scarmeas N, Torgan R, et al. Clinical characteristics and longitudinal changes of informal cost of Alzheimer’s disease in the community. J Am Geriatr Soc. 2006;54:1596–1602. | |
Zhu CW, Scarmeas N, Torgan R, et al. Longitudinal study of effects of patient characteristics on direct costs in Alzheimer disease. Neurology 2006;67:998–1005. | |
Stern Y, Albert SM, Sano M, et al. Assessing patient dependence in Alzheimer’s disease. J Gerontol. 1994;49(5):M216–M222. | |
Murman DL, Von Eye A, Sherwood PR, Liang J, Colenda CC. Evaluated need, costs of care, and payer perspective in degenerative dementia patients cared for in the United States. Alzheimer Dis Assoc Disord. 2007;21:39–48. | |
Gillespie P, O’Shea E, Cullinan J, et al. The effects of dependence and function on costs of care for Alzheimer’s disease and mild cognitive impairment in Ireland. Int J Geriatr Psych. 2013;28(3):256–264. | |
Gustavsson A, Cattelin F, Jönsson L. Costs of care in a mild-to-moderate Alzheimer clinical trial sample: key resources and their determinants. Alzheimers Dement. 2011;7(4):466–473. | |
Rapp T, Andrieu S, Molinier L, et al. Exploring the relationship between Alzheimer’s disease severity and longitudinal costs. Val Health. 2012;15(3):412–419. | |
Knapp M, King D, Romeo R, Sato A, Jones RW, Lacey LA for the DADE Investigator Group. Relationship between patient dependence and health and social care and informal care costs: results from the dependence in AD in England (DADE) study. Poster presented at: The 16th Congress of the European Federation of Neurological Societies (EFNS); September 8–11; 2012; Stockholm, Sweden. | |
American Psychological Association (APA). Diagnostic and Statistical Manual of Mental Disorders. Revised 4th ed. Washington, DC: American Psychiatric Press; 1994. | |
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. | |
Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. | |
Wimo AW, Winblad B. Resource utilization in dementia: RUD Lite. Brain Aging. 2003;3(1):48–59. | |
Instituto Nacional de Estadística (INE). índice de precios de consumo. Available from: http://www.ine.es/. Accessed September 2, 2013. | |
Wimo A, Jönsson L, Bond J, Prince M, Winblad B; Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1. e3–11. e3. | |
Salvi F, Miller MD, Grilli A, et al. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc. 2008;56(10):1926–1931. | |
Zhu CW, Leibman C, McLaughlin T, et al. Patient dependence and longitudinal changes in costs of care in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;26(5):416–423. | |
Zhu CW, Leibman C, McLaughlin T, Scarmeas N, et al. The effects of patient function and dependence on costs of care in Alzheimer’s disease. J Am Geriatr Soc. 2008;56(8):1497–1503. | |
Corder LS, Woodbury MA, Manton KG. Proxy response patterns among the aged: effects on estimates of health status and medical care utilization from the 1982–1984 long-term care surveys. J Clin Epidemiol. 1996;49:173–182. | |
Neumann PJ, Araki SS, Gutterman EM. The use of proxy respondents in studies of older adults: lessons, challenges, and opportunities. J Am Geriatr Soc. 2000;48:1646–1654. | |
McDaid D. Estimating the costs of informal care for people with Alzheimer’s disease: methodological and practical challenges. Int J Geriatr Psychiatry 2001;16:400–405. | |
Karlsson G, Jonsson B, Wimo A, Winblad B. Methodological issues in health economics of dementia. In: Wimo A, Jonsson B, Karlsson G, Winblad B, editors. Health Economics of Dementia. London: Wiley; 1998:161–169. | |
Diario Oficial de Galicia Núm. 213 Martes, 8 de noviembre de 2011 Available from: http://www.xunta.es/dog/Publicados/2011/20111108/AnuncioC3K1-041111-8283_es.html. Accessed September 2, 2013. | |
Diari Oficial de la Generalitat de Catalunya Núm 5907, de 27.06.2011. Available from: http://www20.gencat.cat/portal/site/portaldogc. Accessed September 2, 2013. | |
Boletín Oficial del Principado de Asturias Núm. 80 de 6-iv-2011 Pág. 1/87 Available from: https://sede.asturias.es/portal/site/Asturias/menuitem. 048b5a85ccf2cf40a9be6aff100000f7/?vgnextoid=c0c756a575acd010VgnVCM100000bb030a0aRCRD. Accessed September 2, 2013. | |
Osakidetza. Tarifas para facturación de servicios sanitarios y docentes de osakidetza para el año 2013. Available from: http://www.osakidetza.euskadi.net/r85ekgnrl00/es/contenidos/informacion/libro_tarifas/es_libro/adjuntos/tarifas2013.pdf. Accessed September 2, 2013. | |
Instituto Nacional de Estadística. Encuesta de Estructura Salarial 2010. Available from: http://www.ine.es/prensa/np741.pdf. Accessed September 2, 2013. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





