Back to Journals » Patient Preference and Adherence » Volume 8
Relationship between medication beliefs, self-reported and refill adherence, and symptoms in patients with asthma using inhaled corticosteroids
Authors Van Steenis MNA, Driesenaar JA, Bensing JM, Van Hulten R, Souverein PC , Van Dijk L , De Smet PAGM, Van Dulmen AM
Received 18 February 2013
Accepted for publication 7 May 2013
Published 13 January 2014 Volume 2014:8 Pages 83—91
DOI https://doi.org/10.2147/PPA.S44185
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
MNA Van Steenis,1 JA Driesenaar,2 JM Bensing,2,3 R Van Hulten,4 PC Souverein,4 L Van Dijk,2,4 PAGM De Smet,5 AM Van Dulmen2,6,7
1Department of Pharmaceutical Sciences, Utrecht University, Utrecht, The Netherlands; 2NIVEL (Netherlands institute for health services research), Utrecht, The Netherlands; 3Department of Psychology, Faculty of Social and Behavioural Sciences, Utrecht University, Utrecht, The Netherlands; 4Division of Pharmacoepidemiology and Clinical Pharmacology, Utrecht University, Utrecht, The Netherlands; 5IQ Healthcare, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands; 6Department of Primary and Community Care, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands; 7Department of Health Sciences, Buskerud University College, Drammen, Norway
Background: Beliefs play a crucial role in medication adherence. Interestingly, the relationship between beliefs and adherence varies when different adherence measures are used. How adherence, in turn, is related to asthma symptoms is still unclear. Our aim was to investigate the relationship between beliefs (ie, necessities and concerns) about inhaled corticosteroids (ICS) and subjectively as well as objectively measure adherence and the agreement between these measures. Further, the relationship between adherence and asthma symptoms was examined.
Methods: A total of 280 patients aged 18–80 years who filled at least two ICS prescriptions in the preceding year were recruited to complete a questionnaire. The questionnaire included the Beliefs about Medicines Questionnaire to assess necessity beliefs and concerns about ICS, four questions about ICS use to measure self-reported adherence, and the Asthma Control Questionnaire to assess asthma symptoms. Proportion of days covered was used to determine pharmacy refill adherence.
Results: Data from 93 patients with asthma were analyzed. Necessities were positively related to self-reported adherence (P = 0.01). No other associations were found between beliefs and subjective or objective adherence. There was no correlation between self-reported and refill adherence. Participants were significantly (P < 0.001) less adherent according to self-report data (24.4%) than according to pharmacy data (57.8%). No relationship was found between adherence and asthma symptoms.
Conclusion: Higher necessities are associated with higher self-reported adherence, suggesting that it could be more important to focus on necessities than on concerns in an attempt to improve adherence. Self-reported and refill adherence measurements cannot be used interchangeably. No relationship between adherence and asthma symptoms was found.
Keywords: asthma, inhaled corticosteroids, adherence, medication beliefs, asthma symptoms
Introduction
Asthma affects approximately 520,000 people in The Netherlands1 and is an increasing public health concern worldwide.2 The goal in treating asthma is achieving and maintaining symptom control with a minimum number of drugs. Inhaled corticosteroids (ICS) are the most effective anti-inflammatory medications in treating asthma, and are used as first-line therapy in its long-term or even lifelong treatment.3–6 Unfortunately, ICS adherence, ie, the degree to which patients take their ICS as prescribed by their care provider7,8 seems to be very poor (approximately 50%).9,10
There are several factors related to medication adherence,11–14 of which patients’ beliefs about medication are considered an important aspect in their motivation to take the medication as prescribed.15 Specific beliefs are related to thoughts about the patient’s own medication and appear to be more strongly associated with medication adherence than general beliefs about medication.16 Two specific medication beliefs can be distinguished according to the often used framework developed by Horne et al,17 ie, necessities and concerns. Patients can have specific thoughts related to the necessity of their medication in maintaining their health. On the other hand, patients can also have specific feelings (concerns) about the possible harmful long-term effects and dependence on their medication.
Numerous types of measurement exist to assess adherence, ie, direct, indirect, subjective, and objective methods.18 Menckeberg et al9 have already demonstrated that beliefs about ICS correlate with both self-reported adherence and refill adherence. In a group of patients aged 18–45 years, higher concerns were correlated with lower self-reported adherence and higher necessities with higher refill adherence. Because of the great variance in measurements which can be applied to assess adherence, further characterization of the association between (non)adherence and medication beliefs remains relevant.9,19 Furthermore, we do not know whether these relationships can be replicated and whether they also exist in patients older than 45 years.
To some extent, studies have indicated a (noncausal) relationship between adherence with ICS and asthma symptoms, often using a self-reported scale to assess patient adherence.20–23 Given the subjectivity of self-report instruments, it is important to use objective methods as well to determine the relationship between adherence and asthma symptoms. Currently, this relationship has not been fully elucidated.24,25
The aim of this study was to determine the relationship between medication beliefs (ie, necessities and concerns) and adherence with ICS in an adult population aged 18–80 years. In addition, it aimed to examine the association between adherence and asthma symptoms. Finally, objective and subjective methods of measuring adherence were compared which give insight into their agreement.
Materials and methods
Research design and setting
This study is part of a larger research project investigating communication about ICS inhalers in pharmacies. The research proposal was assessed by the medical ethics committee of the University Medical Centre Utrecht. The medical ethics committee concluded that it was unnecessary to assess the proposal according to the law on medical scientific research involving human beings.
This cross-sectional study was conducted between May and July 2011 in one pharmacy situated in The Netherlands. Participants were selected from the pharmacy system using ATC codes (unique codes for each medicine according to the Anatomical Therapeutic Chemical classification system) for ICS and combination products of β2-agonists and ICS.26 Adult patients aged 18–80 years were invited to participate if they had used ICS for at least one year, and had filled at least two ICS prescriptions within the last year. Patients were excluded if they used a combination of medicines together with their ICS (eg, ICS and tiotropium), which indicates chronic obstructive pulmonary disease (COPD) instead of asthma.
Patients and procedure for data collection
A total of 280 patients met the selection criteria and were invited to participate. The sample size was determined by the number of patients in this particular pharmacy who met the inclusion criteria. A questionnaire had to be completed by the participants, and pharmacy data were extracted from the year prior to the inclusion date.
Participants were recruited by sending an information package with an information letter, an informed consent form, a questionnaire, and a return envelope. The front page of the questionnaire was marked with a sticker with the name of the ICS used by the patient. This made clear to patients that the questions were about their anti-inflammatory drugs (ICS) and not about other (inhaled) medicines.
Measurement instruments
The questionnaire included questions about sociodemographics (ie, age, gender, and education). In addition, questions were asked about smoking and sport habits and the indication for ICS prescription for asthma (symptoms), COPD, not known. Furthermore, two questions about ICS inhaler use (ICS use/day and puffs of ICS/time) were included.
ICS adherence was measured as self-reported adherence and as refill adherence. Self-reported adherence was measured using a scale with four dichotomous items comparable but not identical to the items from the Morisky scale.27 This scale was used to determine medication adherence in a subjective way and consists of four questions which can only be answered with yes (0 point) or no (1 point). An example of the questions is “Are you careless sometimes about taking your medicine?” Scores can be added up to generate a score range of 0–4. A score closer to four indicates higher adherence. Participants were divided into two groups, ie, medication adherent (score of 4) and medication nonadherent (score <4).28 In addition, the scores were also used as continuous data. Pharmacy dispensing data for ICS were used to determine objectively measured refill adherence by calculating the proportion of days covered29 by dividing the total of one day’s supply by the total number of days evaluated, multiplied by 100%. The evaluation period for every person was about 365 days (one year). Episodes of medication use were truncated if the medication gap was ≥182 days (half a year). After calculation of refill adherence via pharmacy data, participants were divided into adherent users and nonadherent users. Patients were classified as nonadherent at the commonly used cutoff point of ≤80%.30–32
The validated Beliefs About Medicines Questionnaire (BMQ-specific) was used to assess specific ICS beliefs.16 The BMQ consists of a necessity scale and a concerns scale, each containing five 5-point Likert scaled items, ranging from “strongly disagree” to “strongly agree”. An example of a concerns item is “Having to take medicines worries me” and of a necessity item “My health in the future will depend on my medicines”. The scores were added up for both scales to produce a score ranging from 5 to 25. Higher scores indicate stronger beliefs. To determine which of the two scales was most important for the participants, a necessity-concerns differential was calculated by subtracting concerns scores from necessity ones, leading to a score range of -20 to 20. Lower scores indicate lower perceived necessity, which suggests more negative feelings towards using ICS medication.9,33 To assess the association between medication beliefs and adherence from a more categorical perspective, four categories were created: patients with low necessities and high concerns, patients with low necessities and low concerns, patients with high necessities and high concerns, and patients with high necessities and low concerns. These groups can be classified as skeptical, indifferent, ambivalent, and accepting, respectively.9,34 To determine low/high necessity/concerns, the scale midpoint (indicated as 15) was used as the cutoff.
The validated, six-item Asthma Control Questionnaire (ACQ) without Lung Function was used to assess asthma control,35,36 rated on a 7-point Likert scale from “no impairment” (0 points) to “maximum impairment” (6 points). An example of a question is “On average, during the past week, how often were you woken by your asthma during the night?” All items are added up and divided by six; a score of 1.5 or higher was regarded as not well controlled asthma.36,37
Data analysis
Pharmacy and questionnaire data were manually transferred into a Statistical Package for the Social Sciences version 17.0 database (SPSS Inc, Chicago, IL, USA). A one-sample test of proportions was used to determine whether patients were more or less adherent according to self-reported or refill adherence. A Pearson’s Chi-square test was used to determine associations between refill adherence and self-reported adherence, and between refill/self-reported adherence and BMQ attitudinal group. Multiple regression analyses were performed, all adjusting for age, gender, educational level, and exercise habits. Linear and logistic regression analyses of necessities and concerns on self-reported adherence were done. Logistic regression analyses were carried out to assess the relationship between medication beliefs and refill adherence, and the association between asthma control and self-reported and refill adherence, respectively.
Results
Participants
Questionnaires were returned by 142 of 280 patients (response rate 50.7%). Seven questionnaires were returned as “wrongly addressed” (net sent rate, 96.8%). Of the 142 respondents, 93 reported having asthma, 21 reported having COPD, and 12 suffered from both asthma and COPD. Sixteen respondents reported their health problem as unknown or did not complete the question (missing). For the purpose of this study, only the questionnaires of patients with asthma without COPD (n = 93) were analyzed. Three-quarters of the participants were highly educated. Approximately, half of the participants was aged 18–44 years and the other half was 45 years or older (Table 1).
Refill and self-reported adherence
The mean refill adherence rate was 79.1% ± 17.2%, ranging from 38.4% to 100.0% (n = 90). Table 2 illustrates the distribution of participants over the self-reported adherence scores (ie, times answered “no” to a question). A higher score indicates a higher adherence rate. Almost a quarter of the patients (24.4%) answered “no” to all questions. However, none of the participants stopped using their medicine when they felt worse when taking it, so no participant did not answer “no” at all. Most participants (65.6%) forgot their medicine at least once in the preceding month (data not shown).
  | Table 2 Distribution for patient self-reported adherence scores using the self-reported adherence scale |
A one-sample test of proportions showed that participants were significantly less adherent according to self-report (24.4% adherent) than according to pharmacy data (57.7% adherent) (P < 0.001). Moreover, no association existed between self-reported adherence and refill adherence (Table 3). Only 50% (15.9% + 34.1%) of the participants were classified in the same adherence category according to subjective and objective adherence measurements. The mean refill adherence rate in self-reported adherent participants did not differ significantly from self-reported nonadherent participants.
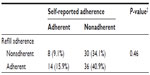  | Table 3 Participants classified as (non)adherent according to self-report or pharmacy data (n = 88, five missing) |
Medication beliefs
The internal consistency of the necessity scale of the BMQ was good (Cronbach’s α = 0.81) and the consistency of the concerns scale was moderate (Cronbach’s α = 0.65). More than one third of the participants (39.9%) had doubts about the necessity of using ICS (scores below scale midpoint). Approximately three-quarters (76.7%) indicated few concerns about using ICS (scores below scale midpoint). Participants scored higher on the necessity scale than on the concerns scale (necessity-concerns difference 3.77 ± 4.37, ranging from -8 to 15), which indicates a positive evaluation towards use of ICS medication.
Beliefs about ICS and adherence
Figure 1 shows the distribution of participants over the four BMQ categories with percentage of adherent participants within each group. Most participants reported high necessities and low concerns (accepting, n = 38; 43.7%), whereas the skeptical group (low necessities, high concerns) consisted of only five participants (5.7%) only. The percentage of adherent patients according to self-report was lowest in the indifferent group (13.3%) and skeptical group (0.0%) and highest in the accepting group (33.3%). According to refill adherence, the percentage of adherent patients was highest in the skeptical group (80.0%); however, the number of participants in this group is very low. Refill adherence is also high in the accepting group (63.2%), followed by the indifferent group (55.2%) and ambivalent group (40.0%). No associations between BMQ category and, on the one hand, self-reported adherence, and, on the other hand, refill adherence were found.
Multiple linear regression analysis revealed a significant positive association (P = 0.01) between necessities and self-reported adherence, adjusted for confounders (Table 4). Multiple logistic regression analysis confirmed this association (P = 0.02, data not shown). Table 5 illustrates that necessities are higher (P = 0.01) in participants classified as self-reported adherent (17.1 ± 3.3) than in those classified as self-reported nonadherent (14.5 ± 4.3). However, no association was found between necessities and refill adherence. The regressions of self-reported (linear) and refill adherence (logistic) regarding concerns did not show a significant relationship, so there was no association between concerns and adherence.
  | Table 5 Beliefs in refill/self-reported adherent and nonadherent participants |
Adherence and asthma symptoms
Internal consistency of the ACQ was good (Cronbach’s α = 0.84). Most participants (84.6%) had well controlled asthma (ACQ <1.5). No significant associations were found between self-reported or refill adherence and asthma control. Furthermore, logistic regression analyses of necessities and concerns about asthma control did not reveal an association.
Discussion
Relationship between beliefs about ICS and adherence
According to the BMQ scores, participants’ beliefs regarding concerns and necessities were favorable with regard to the use of ICS because scores on the necessity scale were high and those on the concerns scale relatively low. According to these results, high adherence rates would be expected, because high needs and low concerns increase adherence.9,13,38 Nevertheless, this was not found in this study. Self-reported adherence was low, and although refill adherence was over two times higher than self-reported adherence, no association between beliefs and refill adherence was found.
Menckeberg et al9 showed that higher necessities were correlated with higher refill adherence and also showed a correlation between concerns and self-reported adherence. The present study only found a positive association between self-reported adherence and necessities. This is consistent with findings in other studies, in which a stronger belief in the necessity of medication was also a predictor for higher adherence.39,40
Most participants reported low concerns and high necessities, and were classified as “accepting” according to the four attitudinal types. In this group, the self-reported adherence level was the highest, although no significant differences in adherence rates were found between the four groups. No significant association between the attitudinal groups and adherence was seen either.
Self-reported adherence levels were highest in the accepting and ambivalent group, which is similar to the findings of Menckeberg et al.9 In contrast, the present study shows the highest refill adherence rates in the accepting and skeptical group. However, adherence rates in the skeptical group are less reliable, since only five participants were classified in this group.
Relationship between adherence and asthma symptoms
Consistent with the findings of Menckeberg et al,9 adherence with ICS was not associated with asthma control. This is in contrast with a study by Clatworthy et al,20 which showed an association between not well controlled asthma and low self-reported adherence with ICS. A possible explanation for this is that only a small proportion of the participants did not have well controlled asthma (15.4%).
Even though a positive relationship between adherence and asthma symptoms would be expected, well controlled asthma could also lead to less ICS use. Since patients could experience a low need for ICS when not suffering from clinically relevant symptoms, this could lead to nonadherence as well.41,42
Relationship between self-reported and refill adherence
This study showed no association between self-reported adherence and refill adherence (continuous as well as dichotomous self-reported adherence) to ICS. Only half of the participants were classified in the same group according to subjective and objective ICS adherence. Approximately two-thirds of the participants who were classified as adherent based on pharmacy data were classified as nonadherent according to self-report. This could be due to the relatively strict classification of adherent/nonadherent participants via self-report. Answering “yes” to the question “Do you ever forget to take your medicine?” on the self-reported adherence scale made a participant nonadherent. Classification as adherent/nonadherent according to pharmacy data is less strict; even if a participant misses up to 20% of their medication, the participant is still regarded as adherent. This allows participants to be classified as adherent even if they behave nonadherently in some way. Besides, even if a participant is adherent according to refill adherence, it is still questionable whether the medicine is actually taken. A prescription can be filled at the pharmacy, but it is unknown what happens thereafter. This problem is not present with self-reported adherence.
It is very important how the threshold in adherence/nonadherence is established, because this is the basis on which conclusions are drawn. This is why a post hoc analysis was performed in which the threshold of the self-reported adherence scale was changed from a score of 4 as adherent to a score of ≥3 as adherent, after which the classification in adherence changed. Another reason to perform this analysis is that mean refill adherence in self-reported adherent participants did not differ from self-reported nonadherent participants. Self-reported adherence shifted from 22 adherent participants to 47 adherent participants (52.2%). Using this format, self-reported adherent participants had a mean refill adherence of 82.5% ± 15.9%, which is significantly higher (P = 0.03) than the mean refill adherence of 74.7% ± 17.9% of the nonadherent participants according to self-report. This is an additional indication that the threshold of the self-reported adherence scale used divides people into nonadherent/adherent in a stricter manner than does refill adherence.
To provide an easy method for filling in the questions on the self-reported adherence scale, the instruction of the questionnaire indicated that the statements that had to be completed were regarding the preceding month. The consequence of this is that it is more difficult to compare self-reported adherence with refill adherence, because the latter method covered adherence during the preceding year. However, if the statements referred to the preceding year, potentially even more participants would be classified as self-reported nonadherent.
Refill adherence has more inherent difficulties. First of all, objective adherence measured with pharmacy data can be calculated in different ways.43 Second, many assumptions have to be made in order to calculate refill adherence. This is especially the case for calculations which determine adherence with ICS. For example, sometimes dosage instructions were not clear (eg, 1–2 puffs per day and usage known), and the researchers had to choose the most obvious instruction. In addition, assessment was done using treatment episodes. These introduce bias, because shorter periods (<30 days) result in higher adherence rates and longer periods (>180 days) result in lower adherence rates. In the present study, episodes were defined as ≤182 days (half a year). Self-reported adherence with ICS is not influenced by this problem because it does not depend on episodes. The 80% cutoff for determination of adherent participants is also an assumption. Shifting this cutoff provides a different classification of adherent participants. Altogether, this leads to refill adherence with ICS being a rough estimation. Using both self-reported and refill rates to indicate a person’s nonadherence probably offers the most valid estimation in daily practice.
In this study, no association could be established between medication beliefs and adherence, and, asthma symptoms. These relationships seem to operate in a complex manner, in which each element could influence another in a positive or contrary way. Adherence is expected to enhance asthma control, whereas asthma control could lead to nonadherence. Moreover, asthma symptoms can affect beliefs about medication, which in turn affect adherence with ICS, and consequently asthma symptoms themselves can change. Further research is needed to study these hypotheses.
Limitations
The study population had some unique characteristics, which restricts the ability to extrapolate its results to other populations. An important issue is the high educational level of the participants, in that 75.3% was highly educated, while in the general Dutch asthma population only 24.2% has been classified as highly educated.44 Higher education can lead to nonrepresentative adherence levels, because low levels of education have been associated with poorer adherence to ICS.45
Distinguishing between asthma and COPD in the pharmacy data was done on the basis of using comedication prescribed for COPD, which is not an ideal method. This was shown by a relatively large number of participants (14.8%) who described their health problem as COPD instead of asthma. An additional explanation for this large number could be that not all participants were aware of the exact nature of their health problem. It has been shown that not everybody with asthma actually knows that they have asthma.3
In addition, only data from one pharmacy were used in this study. This could also explain the higher educational level of the study participants, because the particular pharmacy is situated in a wealthier neighborhood in Utrecht, The Netherlands.
Implications for clinical practice
This study showed that higher necessities were associated with higher self-reported adherence. Education about the need for ICS medication could potentially be beneficial in patients with lower perceived needs.8 In this study, 11.3% of respondents did not know the reason for their ICS prescription. This implies that more information should be provided by prescribers as well as pharmacists. This could lead to an improvement in understanding the disease and medication, and better awareness about the need for medication. Interventions by pharmacists which are intended to increase knowledge about medication and disease are indeed known to improve clinical outcomes and are therefore recommended.46–48
Conclusion
We found that higher necessities are related to higher self-reported adherence. This suggests that in order to increase adherence it is more important to focus on strengthening needs than on diminishing concerns. The present study did not find an association between adherence and asthma symptoms. However, based on this study alone it cannot be ruled out that this association does not exist. Finally, there was no relationship between self-reported and refill adherence with ICS. This indicates that it cannot be simply assumed that self-reported adherence is a correct representation of refill adherence. Therefore, it is important to take both measurement methods into account in clinical practice as well as in further research.
Acknowledgment
Data were collected with the help of pharmacists belonging to the Utrecht University Pharmacy Practice Research (UPPER) network, and the work was conducted in compliance with the requirements of the UPPER institutional review board at the Department of Pharmacoepidemiology and Pharmacotherapy (http://www.uu.nl/vkc/upper).
Disclosure
None of the authors have any conflicts of interest to declare in this work.
References
LAN [Lung Alliance The Netherlands]. Goed gebruik inhalatiemedicatie astma en COPD [Proper use inhalation medication asthma and COPD]. 2011. Available from: http://www.longalliantie.nl/publicaties-. Accessed June 12, 2013. | |
To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. | |
Meltzer EO. The role of the immune system in the pathogenesis of asthma and an overview of the diagnosis, classification, and current approach to treating the disease. J Manag Care Pharm. 2003;9(Suppl 5):8–13. | |
Scichilone N, Contino A, Figlioli GB, Paglino G, Bellia V. Patient perspectives in the management of asthma: improving patient outcomes through critical selection of treatment options. Patient Prefer Adherence. 2010;4:17–23. | |
Geijer RMM. NHG Standaarden voor de Huisarts. [NHG Standards for General Practioners]. Houten, The Netherlands: Bohn Stafleu van Loghum; 2011. Dutch. | |
Global Initiative for Asthma. From the Global Strategy for Asthma Management and Prevention. 2012. Available from: http://www.ginasthma.org/. Accessed June 12, 2013. | |
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. | |
Horne R. Compliance, adherence, and concordance: implications for asthma treatment. Chest. 2006;130(Suppl 1):65S–72S. | |
Menckeberg TT, Bouvy ML, Bracke M, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res. 2008;64:47–54. | |
Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114:1288–1293. | |
Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh. 2003;35:207. | |
Van Dijk L, Heerdink ER, Somai D, et al. Patient risk profiles and practice variation in nonadherence to antidepressants, antihypertensives and oral hypoglycemics. BMC Health Serv Res. 2007;7:51. | |
Laforest L, El Hasnaoui A, Pribil C, et al. Asthma patients’ self-reported behaviours toward inhaled corticosteroids. Respir Med. 2009;103:1366–1375. | |
Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;2:CD000011. | |
Williams LK, Peterson EL, Wells K, et al. A cluster-randomized trial to provide clinicians inhaled corticosteroid adherence information for their patients with asthma. J Allergy Clin Immunol. 2010;126:225–231. | |
Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1–24. | |
Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining nonadherence to preventer medication. Psychol Health. 1999;14:1–24. | |
Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21:1074–1090. | |
Fialko L, Garety PA, Kuipers E, et al. A large-scale validation study of the Medication Adherence Rating Scale (MARS). Schizophr Res. 2008;100:53–59. | |
Clatworthy J, Price D, Ryan D, Haughney J, Horne R. The value of self-report assessment of adherence, rhinitis and smoking in relation to asthma control. Prim Care Respir J. 2009;18:300–305. | |
Hermosa JL, Sanchez CB, Rubio MC, Minguez MM, Walther JL. Factors associated with the control of severe asthma. J Asthma. 2010;47:124–130. | |
Molimard M, Le Gros V. Impact of patient-related factors on asthma control. J Asthma. 2008;45:109–113. | |
Schatz M. Predictors of asthma control: what can we modify? Curr Opin Allergy Clin Immunol. 2012;12:263–268. | |
Bae YJ, Kim TB, Jee YK, et al. Severe asthma patients in Korea overestimate their adherence to inhaled corticosteroids. J Asthma. 2009;46:591–595. | |
Suzuki T, Saito I, Adachi M, Shimbo T, Sato H. Influence of patients’ adherence to medication, patient background and physicians’ compliance to the guidelines on asthma control. Yakugaku Zasshi. 2011;131:129–138. | |
World Health Organization Collaborating Centre for Drugs Statistics Methodology. ATC/DDD Index 2013. Available from: http://whocc.no/atc_ddd_index. Accessed June 12, 2013. | |
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. | |
George CF, Peveler RC, Heiliger S, Thompson C. Compliance with tricyclic antidepressants: the value of four different methods of assessment. Br J Clin Pharmacol. 2000;50:166–171. | |
Caetano PA, Lam JM, Morgan SG. Toward a standard definition and measurement of persistence with drug therapy: examples from research on statin and antihypertensive utilization. Clin Ther. 2006;28:1411–1424. | |
Erickson SR, Coombs JH, Kirking DM, Azimi AR. Compliance from self-reported versus pharmacy claims data with metered-dose inhalers. Ann Pharmacother. 2001;35:997–1003. | |
Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. | |
Guenette L, Moisan J, Preville M, Boyer R. Measures of adherence based on self-report exhibited poor agreement with those based on pharmacy records. J Clin Epidemiol. 2005;58:924–933. | |
Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–567. | |
Mann DM, Ponieman D, Leventhal H, Halm EA. Predictors of adherence to diabetes medications: the role of disease and medication beliefs. J Behav Med. 2009;32:278–284. | |
Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. | |
Sastre J, Olaguibel J, Vega JM, Del Pozo V, Picado C, Lopez Viña A. Cut-off points for defining asthma control in three versions of the Asthma Control Questionnaire. J Asthma. 2010;47:865–870. | |
Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621. | |
Ulrik CS, Backer V, Soes-Petersen U, Lange P, Harving H, Plaschke PP. The patient’s perspective: adherence or nonadherence to asthma controller therapy? J Asthma. 2006;43:701–704. | |
Barber N, Parsons J, Clifford S, Darracott R, Horne R. Patients’ problems with new medication for chronic conditions. Qual Saf Health Care. 2004;13:172–175. | |
Byrne M, Walsh J, Murphy AW. Secondary prevention of coronary heart disease: patient beliefs and health-related behaviour. J Psychosom Res. 2005;58:403–415. | |
Menckeberg TT, Bouvy ML, Bracke M, Hugtenburg JG, Lammers JW, Raaijmakers JA. Patients’ understanding of the reasons for starting and discontinuing inhaled corticosteroids. Br J Clin Pharmacol. 2008;66:255–260. | |
Janson SL, Earnest G, Wong KP, Blanc PD. Predictors of asthma medication nonadherence. Heart Lung. 2008;37:211–218. | |
Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. | |
Heijmans MJWM, Rijken PM. Kerngegevens Zorg – 2003, Monitor Zorg-en Leefsituatie van mensen met astma en mensen met COPD. [Monitor care and living situation of people with asthma and people with COPD]. NIVEL. Dutch. | |
Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1810–1817. | |
Barbanel D, Eldridge S, Griffiths C. Can a self-management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax. 2003;58:851–854. | |
Cordina M, McElnay JC, Hughes CM. Assessment of a community pharmacy-based program for patients with asthma. Pharmacotherapy. 2001;21:1196–1203. | |
Narhi U, Airaksinen M, Tanskanen P, Enlund H. The effects of a pharmacy-based intervention on the knowledge and attitudes of asthma patients. Patient Educ Couns. 2001;43:171–177. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



