Back to Journals » Clinical Ophthalmology » Volume 8
Prospective unmasked randomized evaluation of the iStent inject® versus two ocular hypotensive agents in patients with primary open-angle glaucoma
Authors Fea AM , Belda JI, Rękas M , Jünemann A, Chang L, Pablo L, Voskanyan L, Katz LJ, Giamporcaro JE
Received 31 December 2013
Accepted for publication 11 February 2014
Published 7 May 2014 Volume 2014:8 Pages 875—882
DOI https://doi.org/10.2147/OPTH.S59932
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Antonio M Fea,1 Jose I Belda,2 Marek Rękas,3 Anselm Jünemann,4 Lydia Chang,5 Luis Pablo,6 Lilit Voskanyan,7 L Jay Katz8
1Università degli Studi di Torino, Clinica Oculistica, Torino, Italy; 2Hospital Torrevieja Salud, UTE, Servicio de Oftalmologia, Alicante, Spain; 3Military Institute of Medicine, Department of Ophthalmology, Warsaw, Poland; 4Universitätsaugenklinik, Erlangen, Germany; 5Hinchingbrooke Hospital NHS Trust and Moorfields Bedford Hospital NHS Trust, United Kingdom; 6Hospital Universitario Miguel Servet, Servicio Oftalmologico, Zaragoza, Spain; 7SV Malayan Ophthalmological Center, Yerevan, Armenia; 8Wills Eye Institute, Jefferson Medical College, Philadelphia, PA, USA
Purpose: The purpose of this study was to compare outcomes of subjects with open-angle glaucoma (OAG) not controlled on one medication who underwent either implantation of two iStent inject® trabecular micro-bypass devices or received medical therapy consisting of a fixed combination of latanoprost/timolol.
Patients and methods: Of 192 subjects who qualified for the study and were enrolled, 94 were randomized to surgery with implantation of two iStent inject® devices in the treated eye and 98 to receive medical therapy.
Results: At the month 12 visit, 94.7% of eyes (89/94) in the stent group reported an unmedicated intraocular pressure (IOP) reduction of ≥20% versus baseline unmedicated IOP, and 91.8% of eyes (88/98) in the medical therapy group reported an IOP reduction ≥20% versus baseline unmedicated IOP. A 17.5% between-group treatment difference in favor of the iStent inject group was statistically significant (P=0.02) at the ≥50% level of IOP reduction. An IOP ≤18 mmHg was reported in 92.6% of eyes (87/94) in the iStent inject group and 89.8% of eyes (88/98) in the medical therapy group. Mean (standard deviation) IOP decreases from screening of 8.1 (2.6) mmHg and 7.3 (2.2) mmHg were reported in the iStent inject and medical therapy groups, respectively. A high safety profile was also noted in this study in both the iStent inject and medical therapy groups, as measured by stable best corrected visual acuity, cup-to-disc ratio, and adverse events.
Conclusion: These data show that the use of iStent inject is at least as effective as two medications, with the clinical benefit of reducing medication burden and assuring continuous treatment with full compliance to implant therapy as well as having a highly favorable safety profile.
Keywords: ab interno, intraocular pressure, trabecular bypass, OAG, IOP reduction
Introduction
Glaucoma, a debilitating and prevalent disease, is a leading cause of blindness worldwide. The management of glaucoma requires chronic, life-long treatment with a spectrum of therapeutic options, including medications, laser treatment, and surgical implants, with the common goal among these therapies of reducing intraocular pressure (IOP) to a targeted level, preventing loss of visual field due to excessive pressure on the optic nerve, and minimizing the impact on quality of life. Limitations of currently available medical treatments can result in adverse events1 coupled with a lack of patient compliance. These factors have prompted the development of new therapies to preserve visual function by delivering a significant and continued decrease of IOP without compromising patient safety. Use of stents to create a direct route from the anterior chamber to the Schlemm’s canal, thus bypassing the damaged trabecular meshwork, was researched by Spiegel et al.2 Further evolution to this technique was employed in the research and development of micro-invasive glaucoma surgery (MIGS) using ab interno trabecular micro-bypass stents in mild-to-moderate subjects with open-angle glaucoma (OAG). This procedure and use of the first-generation device, the Glaukos iStent® Trabecular Micro-Bypass Stent (Glaukos Corporation, Laguna Hills, CA, USA) was demonstrated to be safe and effective in a prospective, randomized, multicenter US investigational device exemption clinical trial and summarized by Samuelson et al.3 Further validation of the device in longer term (up to 5 years) prospective, randomized trials described by Fea et al, Craven et al, and others have continued to demonstrate the benefits of a single iStent for the reduction of IOP and medication burden.4–8
Implantation of two stents per glaucomatous eye has been evaluated both in vitro and in clinical studies to investigate whether a further increase of outflow can be accomplished. Bahler et al demonstrated in vitro that increased outflow above and beyond that achieved with one stent is a viable option.9 A clinical study by Belovay et al indicated that multiple stents during cataract surgery resulted in a mean reduction in IOP of <15 mmHg coupled with reduced medications through 1 year after implantation.10
To further enable implantation of multiple stents into Schlemm’s canal, a second-generation, smaller, and cone-shaped design (iStent inject® Trabecular Micro-Bypass; Glaukos Corporation) and a modified injector preloaded with two iStent inject devices were developed. This system is now under study in a US pivotal trial. The study by Bahler et al of this newer generation device using a similar method as the previous perfusion-model study showed that the addition of a second stent further increased outflow facility to 0.78±0.66 μL/minute/mmHg.11 Long-term in vivo studies are underway to determine long-term efficacy.
Combination glaucoma drugs enable the possibility of synergistic medical therapies for greater IOP-lowering effect.12–15 Although these drugs may offer IOP reduction with increased compliance over instillation of multiple types of medications, there are still disadvantages, including high cost, inconvenience, potential side effects such as corneal epithelial cell damage, and noncompliance. Taking into consideration the current interest in combination therapies and the usage of multiple stents for IOP-lowering effect, the clinical trial described in this report was proposed. This final report summarizes the safety and efficacy clinical results at 1 year following treatment of 192 subjects randomized to receive either two iStent inject devices or two medications.
Materials and methods
Study design
This trial, also known as the Second Line Study, was conducted at eight investigational sites in six countries (Italy, Spain, Poland, Germany, United Kingdom, and Armenia). The study design was a prospective, randomized trial to compare outcomes of subjects with OAG not controlled on one medication who underwent either implantation of two iStent inject devices or received medical therapy consisting of a fixed combination of latanoprost/timolol (Xalacom®; Pfizer, New York, NY, USA). One-hundred and ninety-two subjects were enrolled and followed for 1 year after treatment. Subjects using one ocular hypotensive medication, who, in the opinion of the investigator, required additional IOP lowering to control their OAG, were screened for the trial and were washed out of their current glaucoma medication in the study eye prior to randomization. This included a 4-week washout for prostaglandin analogs and beta-blockers, or 2-week washout for alpha-adrenergic agonists and carbonic anhydrase inhibitors. Final enrollment criteria were assessed at the baseline visit. In order to qualify, subjects presented with a post-washout IOP between ≥22 mmHg and <38 mmHg. Subjects were then randomized to receive either implantation of two GTS400 stents in the study eye or medical therapy (latanoprost/timolol). Other inclusion criteria included minimum best corrected visual acuity (BCVA) of 20/200 or better, scleral spur clearly visible by gonioscopy, able and willing to attend follow-up visits for 1 year postoperatively, and informed consent.
Subjects were excluded if they were known nonresponders to latanoprost, had secondary glaucoma (with the exception of pseudoexfoliative and pigmentary), prior incisional glaucoma surgery or procedure such as trabeculectomy shunt or collagen implant, cloudy cornea inhibiting gonioscopic view, signs of traumatic or uveitic, neovascular, or angle-closure glaucoma. Prior selective laser trabeculoplasty in the study eye was allowed as long as the procedure was not performed within 90 days prior to the screening visit.
Following the implantation of two stents or initiation of fixed medical therapy, depending on the group assignment, subjects followed an identical schedule of postoperative examinations. Evaluations occurred at day 1, month 1, 3, 6, 9, and 12. IOP was measured between 8–11 am to control for diurnal variation in IOP.
Stent and surgical technique
Subjects randomized to surgery received two iStent inject devices. Each iStent inject model GTS400 is a single-piece heparin-coated, gamma-sterilized titanium stent (Figure 1). An area of reduced outside diameter midway along the device is designed to provide retention within the trabecular meshwork, while multiple outlet lateral lumens (four outflow orifices) are designed to provide an exit route for aqueous humor from the anterior chamber. The stent is symmetrically designed such that it may be used in either the left or right eye. Two GTS400 stents are preloaded in the G2-M-IS injector system (Figure 2). The injector is designed to deliver the stents automatically into Schlemm’s canal when activated by the surgeon. The portion of the injector that enters the eye is a 23-gauge stainless steel tube. The injector features a surgeon-activated release button on the housing, which is pressed to allow the stents to move over a small guiding trocar to exit the injector. Two iStent inject devices were implanted through the trabecular meshwork into Schlemm’s canal at the nasal position, separated by 2–3 clock hours. Following implantation of two iStent inject devices, subjects received topical postoperative anti-inflammatory and anti-infective medications for 4 weeks.
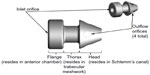  | Figure 1 GTS400 iStent inject® and G2-M-IS injector. |
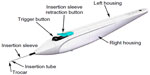  | Figure 2 G2-M-IS injector. |
The study was initiated using the first generation G2-0 injector, which allows for insertion of one stent at a time. Subsequently, the second-generation injector G2-M-IS system, which is able to hold two stents, was introduced to the study, thus providing the clinician the ability to insert multiple stents while entering the eye only once.
Study outcomes and statistical analysis
Efficacy measures included percentage of subjects who achieved an IOP reduction ≥20% versus baseline unmedicated IOP, percentage of subjects who achieved an IOP ≤18 mmHg, mean IOP at each study visit, and mean reduction in IOP. Safety measures assessed cup-to-disc (CD) ratio, BCVA, and incidence of adverse events.
For the proportional analyses such as IOP reduction ≥20% and IOP ≤18 mmHg, exact 95% confidence intervals based on a binomial distribution were calculated for the responder rates. For the iStent inject eyes, responders included eyes on no medication at Month 12. For both groups, a nonresponder assumption was used for missing data. Fisher’s exact test was used to compare the responder rates between the two study groups. For continuous variables such as mean IOP and IOP reduction, mean and standard deviation (SD) were provided. Statistical tests were performed using SAS® software version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
Demographics, preoperative characteristics, and medical therapy
Subject accountability is shown in Table 1. Of the 229 subjects who were screened for the trial, 192 qualified and were enrolled. As shown in Table 2, of the 192 subjects, 94 underwent surgery with implantation of two iStent inject devices in the treated eye and 98 were randomized to receive medical therapy.
  | Table 1 Subject accountability |
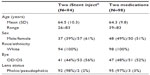  | Table 2 Demographics |
Demographics and subject characteristics were similar for both study arms (Table 2). Mean age for the iStent inject group was 64.5±10.3 years versus 64.3±9.8 years for the group receiving medical therapy. Of the 94 subjects in the iStent inject group, 61% were female and 39% were male versus 51% female and 49% male for the medical therapy group. All subjects in both groups were Caucasian. The majority of eyes in both groups were phakic (98% versus 97% for the iStent inject and medication groups, respectively). No subjects enrolled in the trial had undergone prior selective laser trabeculoplasty. As an alternative to the use of Xalacom (latanoprost/timolol), eight subjects were administered with Duotrav® (travoprost/timolol; Alcon, Inc., Hünenberg, Switzerland), a medication similar to Xalacom in mechanism of action. Four eyes in the iStent inject group were taking medication at the month 12 examination.
Efficacy
At the month 12 visit, 94.7% of eyes (89/94) in the iStent inject group reported an unmedicated IOP reduction ≥20% versus baseline unmedicated IOP, and 91.8% of eyes (88/98) in the medical therapy group reported an IOP reduction ≥20% versus baseline unmedicated IOP (Figure 3). A 17.5% between-group treatment difference in favor of the iStent inject group was statistically significant (P=0.02) at the ≥50% level of IOP reduction. An IOP ≤18 mmHg was reported in 92.6% of eyes (87/94) in the stent group and 89.8% of eyes (88/98) in the medical therapy group (Figure 4).
At month 12, mean IOP in the iStent inject group was 13.0 (SD 2.3) mmHg versus 21.1 (SD 1.7) mmHg at screening and 25.2 (SD 1.4) mmHg at baseline. A mean IOP decrease from screening of 8.1 (SD 2.6) mmHg was reported in the consistent cohort of subjects followed through month 12 (Table 3). For eyes in the medical therapy group, mean IOP at month 12 was 13.2 (SD 2.0) mmHg versus 20.7 (SD 1.7) mmHg at screening and 24.8 (SD 1.7) mmHg at baseline. A mean IOP decrease from screening of 7.3 (SD 2.2) mmHg was reported in the consistent cohort of subjects followed through month 12.
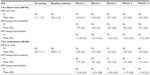  | Table 3 Mean intraocular pressure and intraocular pressure change by visit – all eyes |
Safety measurements
Vertical CD ratio data are provided in Table 4. The proportion of subjects with a CD ratio increase or decrease from preoperative data at month 12 was similar within groups and between groups, and suggests that the CD ratio did not change over the 12-month timeframe. The CD ratio was maintained through month 12 in most eyes. The proportion of eyes with BCVA of 20/40 or better was 84% preoperatively versus 79% at month 12 in the iStent inject group and 87% preoperatively versus 84% at month 12 in the medication group. Five subjects in the iStent inject group and nine subjects in the medication group experienced a slight decrease in BCVA; however, this was anticipated in this population which included eyes with progression of preexisting cataract and other ocular problems.
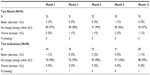  | Table 4 Vertical cup-to-disc ratio change from baseline |
Adverse events and other postoperative observations
Ocular adverse events and other postoperative observations are summarized in Table 5. One adverse event was reported in the iStent inject group – one subject experienced IOP decompensation with an elevated IOP (48 mmHg). The subject was treated with medication and the IOP was lowered to 25 mmHg. One subject had one stent reported as not visible, which was resolved after neodymium-doped yttrium aluminum garnet laser treatment to remove an apparent obstruction. One subject reported soreness/discomfort that was resolved following treatment with nonsteroidal anti-inflammatory medications. Two adverse events were reported in two subjects in the medical therapy group – mild burning of the eye (suspected intolerance to Xalacom) and suspected allergy to medication.
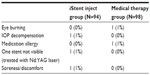  | Table 5 Ocular adverse events and other postoperative observations |
Discussion
A series of studies to treat OAG using the Glaukos micro-bypass iStent technology as a sole procedure was undertaken by the MIGS Study Group. The study group was comprised of surgeons from many countries. Unlike the present multicenter study, these MIGS surgeons performed the procedures at a single site on a homogeneous population in Armenia. One study in the series, to assess the implantation of two iStent devices in subjects with mild-to-moderate OAG not controlled on one preoperative ocular hypotensive medication, reported an average IOP of 13.6 mmHg at 1 year postoperatively without medication and without significant postoperative effects, demonstrating that earlier intervention of patients with mild-to-moderate OAG may potentially be a preferable alternative to chronic use of multiple medications.16 In another study, which assessed two-stent implantation in 41 moderate-to-advanced OAG phakic and pseudophakic patients not controlled on two medications preoperatively, all eyes achieved month 12 IOP reduction ≥20% with reduction of one medication and month 12 IOP ≤18 mmHg with reduction of one medication. Mean IOP reduction >10 mmHg on one medication was sustained through 18 months.17
The Second Line Study, described in this report, was designed in recognition of the European Glaucoma Society guidelines that specify addition of a second medication in primary OAG prior to surgery.18 This pan-European, multicenter study considered surgery with a new MIGS device as an alternative to a second medication under a prospective, randomized study design of the iStent inject device versus a fixed combination of prostaglandin/beta-blocker. A significantly higher proportion of iStent inject eyes versus medication eyes achieved month 12 IOP reduction ≥50% versus baseline IOP. Mean IOP in the iStent inject group at 1 year was 13.0 mmHg versus 13.2 mmHg in the medication group. Mean IOP decrease from baseline (12.2 mmHg in the iStent inject group versus 11.6 in the medication group) was reported. These data show that the use of iStent inject is at least as effective as two medications, with the clinical benefit of reducing medication burden and assuring continuous treatment with full compliance to implant therapy.
A high iStent implant safety profile was also noted in this study, as measured by stable BCVA, CD ratio, and adverse events. The low rate of reported adverse events is consistent with work by Arriola-Villalobos on the first-generation stent, in which one eye experienced visual acuity loss due to macular degeneration and one eye required topical medication for increased IOP, in a series of 19 eyes with follow-up through a mean of 54 months.7 Fea et al’s series of ten iStent subjects and 14 control subjects followed for 56 months reported no adverse events in the treatment group and macular drusen in one subject in the control group.6
This study has several strengths, including that it is a multicenter study conducted in a large number of countries, which provides external validation of results. The use of trabecular micro-bypass stents versus ocular hypotensive medications to control IOP is an important development. Newer drugs, such as actin cytoskeleton agents or rho-associated protein kinase inhibitors, are targeted to alter the compromised trabecular meshwork and improve outflow.19 However, patient compliance with chronic, long-term use of topical medications and the associated side effects has been demonstrably poor and is always suspect. Noncompliance with medical therapy, leading to disease progression and eventually to blindness, may be preventable by implantation of this device.
There are several limitations in this work, including that it was not a masked study due to the disparate forms of therapy (surgery in one group, medication in the other group). Because of the qualifying IOP requirement, lower dispersion of IOP measurement data or regression to the mean may have occurred. However, these limitations are highly unlikely to have altered the findings that two iStent inject devices provide comparable benefits to combination medical therapy for OAG subjects.
Long-term follow-up studies are important in order to evaluate efficacy and adverse effects past a 1-year timeframe. For example, long-term data from Craven et al,4 Fea et al,6 and Arriola-Villalobos et al7 on the first-generation iStent showed sustained IOP reduction and excellent safety through <56 months postoperatively. Because the first- and second-generation trabecular micro-bypass devices are both based on the trabecular bypass principle,2 similar favorable long-term efficacy and safety of the iStent inject is expected. Future studies that can assess the use of the iStent inject for a timeframe >1 year are recommended to assess long-term findings. A future summary of patient questionnaire findings from this study is also recommended as this report was limited to safety and efficacy clinical data. Furthermore, this study was limited to white patients only. As some patients, such as black patients, may exhibit higher resistance to glaucoma medical therapy, it is possible that trabecular micro-bypass stents may be of even greater benefit in some racial groups. It is recommended that future work expands the demographic population so that the benefit of the iStent inject can be further evaluated. In this study, stents were placed in the nasal quadrant because this area features the highest distribution of collector channels.20 The authors envision that future studies can examine optimum placement of stents near the opening of major collector channels using newer imaging technologies that can readily identify these physiological structures.21 Finally, although subjects in the medication group were instructed to follow the postoperative medication regimen (and were provided eye drops at no cost to them), the authors relied on the subjects’ responses that they complied with the protocol-specified medication regimen as evidence that the subjects took their eye drops. However, the substantial IOP reduction after treatment suggests strong compliance by the patients.
Conclusion
The favorable results of this third in a series of studies of the MIGS Study Group confirms that micro-invasive surgery using the iStent inject has the potential to be a valid alternative to medication for first-line therapy for mild-to-moderate OAG. The data presented in this publication represent the status of the patients 1 year after surgery; longer-term studies are underway. This study confirms that the iStent inject is a safe and effective implant procedure with a high benefit-to-risk profile and may be a preferable alternative to chronic use of multiple medications in subjects with OAG.
Acknowledgments
Glaukos Corporation provided study devices, sponsorship for performing this study, editorial assistance in the preparation of this manuscript (by Jeannie Gifford Cecka, Clinical and Regulatory Consultant), and payment of the article processing charges.
Disclosure
Dr Fea received financial support from Glaukos for his work as an investigator in this study and has also received non-study financial support from Glaukos. Drs Belda Sanchis, Rkas, Jünemann, Chang, Pablo and Voskanyan received financial support from Glaukos for their work as investigators in this study. Dr Katz received financial support from Glaukos for his work as a medical monitor in this study and has also received non-study financial support from Glaukos. The authors report no conflicts of interest in this work.
References
Liesegang TJ. Conjunctival changes associated with glaucoma therapy: implications for the external disease consultant and the treatment of glaucoma. Cornea. 1998;17(6):574–583. | |
Spiegel D, Garcia-Feijoo J, Garcia-Sanchez J, Lamielle H. Coexistent primary open-angle glaucoma and cataract: preliminary analysis of treatment by cataract surgery and the iStent trabecular micro-bypass stent. Adv Ther. 2008;25(5):453–464. | |
Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE; US iStent Study Group. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. | |
Craven ER, Katz LJ, Wells JM, Giamporcaro JE; iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: 2-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–1345. | |
Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407–412. | |
Fea AM, Pignata G, Bartoli E, et al. Prospective, randomized, double-masked trial of trabecular bypass stent and cataract surgery vs cataract surgery alone in primary OAG: long-term data. In: Program and abstracts of the XXX Congress of the European Society of Cataract and Refractive Surgeons; September 8–12, 2012; Milan, Italy. | |
Arriola-Villalobos P, Martinez-de-la-Casa J, Diaz-Valle D, Fernandez-Perez C, Garcia-Sanchez J, Garcia-Feijoo J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012;96(5):645–649. | |
Spiegel D, Wetzel W, Neuhann T, et al. Coexistent primary open-angle glaucoma and cataract: interim analysis of a trabecular micro-bypass stent and concurrent cataract surgery. Eur J Ophthalmol. 2009;19(3):393–399. | |
Bahler CK, Smedley GT, Zhou J, Johnson DH. Trabecular bypass stents decrease intraocular pressure in cultured human anterior segments. Am J Ophthalmol. 2004;138(6):988–994. | |
Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed II. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911–1917. | |
Bahler CK, Hann CR, Fjield T, Haffner D, Heitzmann H, Fautsch MP. Second-generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. Am J Ophthalmol. 2012;153(6):1206–1213. | |
Ammar DA, Kahook MY. Effects of benzalkonium chloride- or polyquad-preserved fixed combination glaucoma medications on human trabecular meshwork cells. Mol Vis. 2011;17:1806–1813. | |
Nagai N, Murao T, Oe K, Ito Y, Okamoto N, Shimomura Y. [In vitro evaluation for corneal damages by anti-glaucoma combination eye drops using human corneal epithelial cell (HCE-T)]. Yakugaku Zasshhi. 2011;131(6):985–991. Japanese [with English abstract]. | |
Brignole-Baudouin F, Riancho L, Liang H, Nakib Z, Baudouin C. In vitro comparative toxicology of polyquad-preserved and benzalkonium chloride-preserved travoprost/timolol fixed combination and latanoprost/timolol fixed combination. J Ocul Pharmacol Ther. 2011;27(3):273–280. | |
Alm A, Gunden JW, Kwok KK. Five-year, multicenter safety study of fixed combination latanoprost/timolol (Xalacom) for open-angle glaucoma and ocular hypertension. J Glaucoma. 2011;20(4):215–222. | |
Chang DF. Intraocular pressure reduction and safety outcomes following micro-invasive glaucoma surgery with two trabecular micro-bypass stents in OAG. In: Program and abstracts of the XXXI Congress of the European Society of Cataract and Refractive Surgeons; October 5–9, 2013; Amsterdam, the Netherlands. | |
Garcia-Feijoo J, Martinez de la Casa J. Micro-invasive glaucoma surgery (MIGS) with trabecular micro-bypass stents and postoperative prostaglandin in open-angle glaucoma subjects. In: Program and abstracts of the XXXI Congress of the European Society of Cataract and Refractive Surgeons; October 5–9, 2013; Amsterdam, the Netherlands. | |
Heijl A, Traverso CE, editors. Terminology and Guidelines for Glaucoma. 3rd ed. Leuven: European Glaucoma Society; 2008. | |
Bucolo C, Salomone S, Drago F, Reibaldi M, Longo A, Uva MG. Pharmacological management of ocular hypertension: current approaches and future prospective. Curr Opin Pharmacol. 2013;13(1):50–55. | |
Johnstone M, Martin E, Jamil A. Pulsatile flow into the aqueous veins: manifestations in normal and glaucomatous eyes. Exp Eye Res. 2011;92(5):318–327. | |
Ren J, Gille HK, Wu J, Yang C. Ex vivo optical coherence tomography imaging of collector channels with a scanning endoscopic probe. Invest Ophthalmol Vis Sci. 2011;52(7):3921–3925. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


