Back to Journals » Clinical Ophthalmology » Volume 8
Progressive retinal detachment secondary to juxtapapillary microholes in association with type 3 posterior staphylomas
Authors Dinah C, Vaideanu-Collins D, Steel D
Received 5 November 2013
Accepted for publication 21 January 2014
Published 9 June 2014 Volume 2014:8 Pages 1089—1095
DOI https://doi.org/10.2147/OPTH.S57086
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Christiana B Dinah,1 Daniela Vaideanu-Collins,2 David HW Steel1,3
1Ophthalmology Department, Sunderland Eye Infirmary, Sunderland, 2Ophthalmology Department, James Cook University Hospital, Middlesbrough, UK, 3Institute of Genetic Medicine, Newcastle University, Newcastle Upon Tyne, UK
Purpose: This study describes a novel subtype of retinal detachment occurring in eyes with pathological myopia associated with type 3 posterior staphyloma and discusses the management options.
Methods: We retrospectively reviewed the case notes of seven patients who presented with unilateral symptomatic rhegmatogenous retinal detachment secondary to nasal juxtapapillary microholes.
Results: All seven patients had pathological myopia and an associated peripapillary type 3 posterior staphyloma. They all presented with symptoms of acute posterior vitreous detachment and had progressive retinal detachment. All cases were discovered to have a single juxtapapillary hole less than 1 disc diameter from the optic-nerve head, within areas of nasal chorioretinal atrophy. The microholes were identified intraoperatively in six of seven cases, with one case identified preoperatively on optical coherence tomography. In the four most recent cases, successful retinal reattachment was achieved with vitrectomy and C2F6 gas tamponade. The remaining three cases were managed with vitrectomy and silicone oil.
Conclusion: Seven patients with pathological myopia, type 3 posterior staphyloma, and progressive retinal detachment secondary to juxtapapillary microholes are presented in this paper. High clinical suspicion is required to identify these breaks. Successful retinal reattachment with pars plana vitrectomy and long-acting gas is possible.
Keywords: pathological myopia, pars plana vitrectomy, long-acting gas, pathological myopia
Introduction
Pathologic myopia is often associated with characteristic features including axial elongation, chorioretinal degeneration, and posterior staphyloma.1,2 It is a major risk factor for retinal detachment, associated with a variety of peripheral retinal pathologies. Retinal detachment in pathological myopia can also be associated with posterior breaks, most commonly macular holes and occasionally paravascular breaks associated with abnormally firm vitreoretinal adhesions.3,4 Identification of posterior breaks in highly myopic eyes with retinal detachment can be difficult and the management of them even more so. In particular, the optimal management strategy in cases also associated with posterior staphyloma and retinal pigment epithelium (RPE)/choroidal atrophy has been the subject of much debate. Treatment modalities such as pars plana vitrectomy (PPV) with long-term silicone oil, macular buckles, cryotherapy, and transscleral diathermy5–7 have been described, but the optimum treatment remains unclear. Several peripapillary lesions have been described in high myopia, including optic and conus pits, juxtapapillary holes, and intrachoroidal cavitations.8–11 However, their relationship to symptomatic retinal detachment has not been fully explored.
Herein, we report seven patients with high myopia who presented with progressive and symptomatic rhegmatogenous retinal detachments (RRDs) associated with a distinctive type of posterior break; namely, a round microhole occurring adjacent to the optic nerve and within areas of nasal chorioretinal atrophy associated with one specific type of posterior staphyloma.
Methods
We reviewed all retinal detachment cases presenting to two vitreoretinal specialists at the Sunderland Eye Infirmary from the surgeons’ own databases over the last 15 years and included all cases associated with high myopia and juxtapapillary retinal breaks. “High myopia” was defined as axial length >26 mm associated with fundal changes consistent with pathological myopia. “Juxtapapillary retinal breaks” were defined as breaks within 1 disc diameter (DD) of the optic nerve. We excluded cases associated with traumatic or iatrogenic retinal breaks, macula holes, or tractional retinal detachments. Case notes were reviewed to determine clinical presentation, refractive status, presence and type of posterior staphyloma, presence and type of retinal break, management, and postoperative outcome. Clinical photographs, optical coherence tomography (OCT) images with the Topcon 3D OCT-1000 (Topcon Corporation, Tokyo, Japan), and intraoperative videos were also reviewed, when available.
Results
We identified a total of seven cases of RRD associated with juxtapapillary holes. All identified cases were associated with juxtapapillary holes located nasal to the optic nerve and a specific type of posterior staphyloma; namely, type 3 posterior staphyloma. There were two males and five females, with a mean age of 72.4 years (range: 60–82 years). All seven cases presented with symptoms of acute posterior vitreous detachment (PVD) – that is, floaters and/or flashes of light for ≤3 weeks’ duration associated with mean best-corrected visual acuity at presentation of 6/72 (range: hand movements [HM]-6/9). The mean axial length was 27.15 mm (range: 25.25–29.02) with only one patient having axial lower than 26 (25.25), with refractive status ranging from -2.5 diopters (D) to -14 D. On examination, three cases were pseudophakic at presentation, having had cataract surgery at least 2–10 years previously.
All eyes had fundal findings consistent with pathological myopia. A particularly interesting finding was the presence of type 3 posterior staphyloma in all cases in association with extensive peripapillary atrophy. A Weiss ring was also observed in all patients.
All cases are summarized in Table 1 and diagrammatically in Figure 1. In total, five of seven patients presented with inferior retinal detachments that were macula involving in two of the five. The other two cases presented with a nasal retinal detachment not extending to the ora serrata and a total retinal detachment associated with a choroidal hemorrhage.
  | Figure 1 Fundus diagrams of retinal detachments at presentation and ocular parameters. |
The juxtapapillary holes were usually located superonasal to the optic nerve within peripapillary atrophy (Figure 2), with only one case located inferonasally. The holes were small, measuring between 0.5 and 1.5 times the size of an adjacent retinal vein and in all cases within 1 DD of the disc margin.
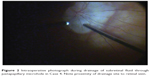  | Figure 2 Intraoperative photograph during drainage of subretinal fluid through juxtapapillary microhole in Case 4. Note proximity of drainage site to retinal vein. |
We were able to obtain a preoperative OCT image in one case (Figure 3), which demonstrated a full-thickness juxtapapillary retinal hole, with an underlying retinal schisis-like cavity and subretinal fluid.
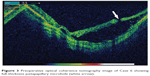  | Figure 3 Preoperative optical coherence tomography image of Case 6 showing full-thickness juxtapapillary microhole (white arrow). |
In all, five patients, including all cases presenting after 2006, had fundus photography and postoperative OCT imaging (Figure 4A–C) undertaken. OCT imaging confirmed posterior staphyloma encompassing the optic nerve (type 3), severely attenuated RPE, and choroid, particularly within peripapillary atrophy (PPA) and the presence of retinalvascular folds within the peripapillary area. Lately, enhanced-depth OCT imaging was performed, confirming these findings.
Our management approach to these cases evolved, so we group them into two periods – before and after 2006. Before 2006, we subscribed to the axiom that retinal detachments in high myopia associated with posterior staphyloma should always be managed using silicone oil as tamponade. As such, the three cases presenting before 2006 were managed with three-port pars plana vitrectomy (3PPV) and silicone-oil tamponade. Endolaser was not typically employed due to the theoretical poor uptake of laser in areas of severe chorioretinal atrophy. After 2006, such cases were managed with 3PPV, endolaser, and long-acting gas tamponade. All four cases were successfully reattached and were still attached at last follow-up.
None of the cases reported here had areas of persistent vitreoretinal adhesion identified at vitrectomy, with triamcinolone staining used to confirm this in the four most recent cases. In the cases for which endolaser was used, adjacent pigmented areas on intact RPE were used as a guide to select the laser power and two rows of confluent laser burns were used to surround the break. The last four cases were treated with C2F6 gas tamponade and were instructed to maintain a face-down posture with temporal cheek to pillow for up to 7 days postoperatively.
Mean follow-up was 17.3 months (range: 6–36 months) and mean best-corrected visual acuity at last follow-up was 6/60 (range: 6/12-counting fingers [CF]).
Discussion
The distinct clinical characteristics in our series included the exclusive association with type 3 posterior staphyloma, occurrence in the nasal region of extensive peripapillary chorioretinal atrophy, and tendency to result in progressive RRD. All cases were preceded by acute signs of PVD and had clinical and surgical signs of PVD. PVD is commonly associated with vitreous traction, resulting in retinal breaks in 10%–15% of eyes. However, in our cohort, these holes were juxtapapillary in location.
There are previous reports of juxtapapillary holes in the literature.11–13 Regenbogen and Stein described seven eyes with juxtapapillary holes that were, in contradistinction to our cases, 2–3 DDs away from the optic nerve and never within peripapillary atrophy.11 In addition, their cases were associated with nonprogressive retinal detachment, even after unsuccessful intervention. Phillips and Dobbie described ten patients with posterior retinal breaks associated with retinal detachment.12 Only one case was associated with a posterior staphyloma and juxtapapillary hole. In that case, the posterior staphyloma encompassed the macular area and the juxtapapillary hole was inferior to the disc. The authors did not describe the presence or extent of chorioretinal degeneration. Adams discussed four cases with small posterior holes in his case series of retinal detachment due to macular and posterior holes.13 In those cases, the described holes were further away from the optic nerve, not specifically associated with chorioretinal degeneration, and associated with a posterior staphyloma in only one case.
Our cases differ from those in the mentioned reports in that they are exclusively related to type 3 posterior staphyloma, exclusively located within areas of peripapillary chorioretinal atrophy, and associated with progressive retinal detachment. As such, we believe retinal detachment due to juxtapapillary microholes in type 3 posterior staphyloma is a specific category and its location within peripapillary chorioretinal atrophy presents unique identification and management challenges.
We can only speculate about the pathogenesis and preferred location of these retinal breaks. Posterior staphyloma are associated with high myopia in up to 90% of cases,14 and highly myopic eyes with posterior staphyloma have a higher probability of visual disturbance.15 In their series, Hsiang et al described increasing severity of posterior staphylomas in high myopes over 50 years of age compared with high myopes under 50 and found increased severity of myopic retinal degeneration with age.14 All cases presented herein involved patients over 50 and are notable for having extensive and severe chorioretinal atrophy predominantly in the nasal peripapillary area.
Type 3 posterior staphyloma is a relatively rare form of staphyloma, occurring in 2.8%–4.3% of high myopes.2,14 In type 3 posterior staphyloma, the staphyloma is centered in a 1.0–2.5 DD radius around the disc, with the disc at its base and often steep margins circumferentially.2 Types 1 and 2 are the most common types of posterior staphyloma among Caucasians,2 and eyes with posterior staphylomas, centered around the fovea (type 2) have been demonstrated to have a higher rate of macula hole-associated retinal detachment.15 Type 4 posterior staphylomas are centered nasal to the optic nerve, encompassing the disc. In our patients, the posterior staphyloma was always centered around the optic nerve, with the optic nerve occupying the deepest part of the staphyloma. We postulate that eyes with type 3 posterior staphyloma are predisposed to peripapillary breaks.
Posterior staphyloma predisposes to retinal detachment in a variety of ways. First, a posterior staphyloma can result in an increase in anteroposterior vitreous traction that could, in areas of vitreoretinal adhesion, result in retinal breaks and macular-hole formation.16 Second, with the relative inelasticity of the retina and, in particular, the inflexibility of retinal vessels,17 the posterior stretching of the sclera can promote separation of the sensory retina from the RPE. In support of this, four out of seven cases described in our series demonstrated retinal vascular microfolds on OCT corresponding to retinal arterioles and venules (Figure 4C). Retinal vascular microfolds are detectable only by OCT and believed to correspond to inward traction by retinal vessels in high myopia.18 We also observed that in all cases the causative retina hole was adjacent to a retinal vein radiating away from the optic disc. It is possible that the inward traction exerted by these peripapillary vessels contributes to juxtapapillary microhole formation and may complicate their management. Third, chorioretinal atrophy within the staphyloma may weaken the adherence between the retina and RPE.19,20 Myopic chorioretinal degeneration is thought to have a predilection for the marginal regions of posterior staphyloma19 and become more severe with increasing depth of posterior staphyloma.14 It is possible that the progression of posterior staphyloma in these patients occurs disproportionately in the nasal margin in association with increasing chorioretinal degeneration, predisposing to microhole formation in the nasal region.
Enhanced-depth OCT has been used to study the choroid. There is evidence that the choroid is thinnest at the edge of a posterior staphyloma and that there may be choroidal vascular abnormalities contributing to serous retinal detachment within posterior staphylomas.21,22 In our series, enhanced-depth OCT was performed in four cases and the choroid was confirmed to be severely attenuated or absent in the area of peripapillary chorioretinal atrophy. We believe the progressive thinning of the retina, RPE, and choriocapillaris in this region in addition to the mechanical forces associated with posterior staphyloma predisposes to the formation of juxtapapillary microholes and progressive retinal detachment.
Retinal detachments with undetected breaks are associated with suboptimal surgical outcomes.23,24 However, the identification of posterior breaks, particularly small ones, can be fraught with difficulty. In our experience, if a diligent preoperative search proves unfruitful, high clinical suspicion of a posterior retinal break should be maintained in cases where the subretinal fluid does not extend to the periphery and where the subretinal fluid has retro-curved anterior borders. Intraoperatively, aspiration over and adjacent to the optic disc may allow detection of protein-rich subretinal fluid draining through the posterior hole, a technique known as “Schlieren flow visualization.”
Management of posterior breaks in high myopia is challenging, as the breaks can be very posterior in location and associated with chorioretinal atrophy and thin sclera. Association with posterior staphyloma introduces more complexity, as the retina must be attached not only along the normal contour of the globe but also along the curvature of the posterior staphyloma. Traditionally, scleral buckling of posterior breaks has been advocated, but all the breaks in this series were located within 2 mm of the edge of the optic nerve and, as such, thought not amenable to buckling because of the risk of adjacent optic-nerve trauma and compression. Using an internal approach, silicone oil has been advocated as the endo-tamponade of choice to provide long-term tamponade to the break in the absence of the ability to form a retinal adhesion between the retina and the area of choroidal/RPE atrophy.25 As such, three of the earlier cases described here were managed with silicone oil, albeit one after failed treatment with long-acting gas. However, silicone oil has a high surface tension and conforms poorly to the globe, which is particularly relevant in cases associated with posterior staphyloma. Transscleral diathermy,7 which is associated with weakening of the already thinned sclera and endocryotherapy26 or endodiathermy,27 both of which (in our experience) can be associated with ganglion cell-layer damage and possible retinal-break enlargement, have been employed by some investigators. Exo-cryotherapy is another option but would be associated with a high risk of optic-nerve damage, given the proximity of the retinal break to the optic nerve. More recently, Spaide and Fisher identified adherent plaques of cortical vitreous in six eyes with high-myopia retinal detachment overlying posterior staphyloma.28 None of the patients in their series was found to have any observable break before or during surgery. They demonstrated successful retinal reattachment by identification and removal of residual cortex with triamcinolone during PPV, followed by endo-tamponade with SF6. Four of the seven cases in our series were stained with triamcinolone and no vitreoretinal traction or plaques were identified.
In our series, PPV with endolaser and C2F6 was employed with success in four cases. The effectiveness of endolaser to create a durable chorioretinal adhesion in this situation is uncertain, as there was no RPE in the area of chorioretinal atrophy to allow for laser uptake. It is possible, however, that some uptake occurs in the retina or sclera; as human sclera (and, to some extent, human retina) has been shown to contain a significant amount of melanin.29,30 Interestingly, Case 7 was treated with silicone oil without endolaser and remained attached after removal of oil 9 years later. It is possible that the long-term use of silicone oil resulted in stretching and molding of the retina, while promoting glial-cell reaction and hole closure, and perhaps the use of long-acting gases with glial-cell stimulation with laser, as we used in the last four cases, had a similar effect.
Conclusion
Herein, we have described seven cases of progressive RRDs in a specific subgroup of patients with pathological myopia and type 3 posterior staphyloma associated with juxtapapillary microholes within nasal chorioretinal atrophy. We were able to achieve long-term success by PPV and endo-tamponade with long-acting gas in the majority of our cases. The limitations of this study include its retrospective nature and small number of cases; however, our observations suggest that patients with type 3 posterior staphyloma are at increased risk of RRD secondary to juxtapapillary microholes. A high degree of clinical suspicion and diligent searching preoperatively and intraoperatively are required to identify and treat these breaks adequately.
Acknowledgment
The authors gratefully acknowledge the help of Mr David Inglesby, FRCOphth, who cared for some of the study patients and with whom we have discussed the manuscript.
Disclosure
The authors declare no conflicts of interest in this work.
References
Curtin BJ. Physiologic vs pathologic myopia: genetics vs environment. Ophthalmology. 1979;86(5):681–691. | ||
Curtin BJ. The posterior staphyloma of pathologic myopia. Trans Am Ophthalmol Soc. 1977;75:67–86. | ||
Stirpe M, Michels RG. Retinal detachment in highly myopic eyes due to macular holes and epiretinal traction. Retina. 1990;10(2):113–114. | ||
Chen L, Wang K, Esmaili D, Xu G. Rhegmatogenous retinal detachment due to paravascular linear retinal breaks over patchy chorioretinal atrophy in pathologic myopia. Arch Ophthalmol. 2010;128(12):1551–1554. | ||
Kuriyama S, Matsumara M, Harada T, Ishigooka H, Ogino N. Surgical techniques and reattachment rates in retinal detachment due to macular hole. Arch Ophthalmol. 1990;108(11):1559–1561. | ||
Matsumura M, Kuriyama S, Harada T, Ishigooka H, Ogino N. Surgical techniques and visual prognosis in retinal detachment due to macular hole. Ophthalmologica. 1992;204(3):122–133. | ||
Bovey E, Gonvers M. Transscleral diathermy: an additional tool in the management of retinal detachment due to posterior breaks in highly myopic eyes. Retina. 1999;19(6):489–494. | ||
Freund KB, Ciardella AP, Yannuzzi LA, et al. Peripapillary detachment in pathologic myopia. Arch Ophthalmol. 2003;121(2):197–204. | ||
Ohno-Matsui K, Akiba M, Moriyama M, et al. Acquired optic nerve and peripapillary pits in pathologic myopia. Ophthalmology. 2012;119(8):1685–1692. | ||
Shimada N, Ohno-Matsui K, Nishimuta A, Tokoro T, Mochizuki M. Peripapillary changes detected by optical coherence tomography in eyes with high myopia. Ophthalmology. 2007;114(11):2070–2076. | ||
Regenbogen L, Stein R. Retinal detachments due to juxtapapillary microholes. Arch Ophthalmol. 1968;80(2):155–160. | ||
Phillips CI, Dobbie JG. Posterior staphyloma and retinal detachment. Am J Ophthalmol. 1963;55:332–335. | ||
Adams ST. Retinal detachment due to macular and small posterior holes. Arch Ophthalmol. 1961;66:528–533. | ||
Hsiang HW, Ohno-Matsui K, Shimada N, et al. Clinical characteristics of posterior staphyloma in eyes with pathologic myopia. Am J Ophthalmol. 2008;146(1):102–110. | ||
Oie Y, Ikuno Y, Fujikado T, Tano Y. Relation of posterior staphyloma in highly myopic eyes with macular hole and retinal detachment. Jpn J Ophthalmol. 2005;49(6):530–532. | ||
Margheria RR, Schepens CL. Macular breaks. 1. Diagnosis, etiology, and observations. Am J Ophthalmol. 1972;74(2):219–232. | ||
Sayanagi K, Ikuno Y, Gomi F, Tano Y. Retinal vascular microfolds in highly myopic eyes. Am J Ophthalmol. 2005;139(4):658–663. | ||
Ikuno Y, Gomi F, Tano Y. Potent retinal arteriolar traction as a possible cause of myopic foveoschisis. Am J Ophthalmol. 2005;139(3):462–467. | ||
Ito-Ohara M, Seko Y, Morita H, Imagawa N, Tokoro T. Clinical course of newly developed or progressive patchy chorioretinal atrophy in pathological myopia. Ophthalmologica. 1998;212(1):23–29. | ||
Morita H, Ideta H, Ito K, Yonemoto J, Sasaki K, Tanaka S. Causative factors of retinal detachment in macular holes. Retina. 1991;11(3):281–284. | ||
Yamagishi T, Koizumi H, Yamazaki T, Kinoshita S. Choroidal thickness in inferior staphyloma associated with posterior serous retinal detachment. Retina. 2012;32(7):1237–1242. | ||
Moriyama M, Ohno-Matsui K, Futagami S, et al. Morphology and long-term changes of choroidal vascular structure in highly myopic eyes with and without posterior staphyloma. Ophthalmology. 2007;114(9):1755–1762. | ||
Wong D, Billington BM, Chignell AH. Pars plana vitrectomy for retinal detachment with unseen retinal holes. Graefes Arch Clin Exp Ophthalmol. 1987;225(4):269–271. | ||
Salicone A, Smiddy WE, Venkatraman A, Feuer W. Management of retinal detachment when no break is found. Ophthalmology. 2006;113(3):393–403. | ||
Chen YP, Chen TL, Yang KR, et al. Treatment of retinal detachment resulting from posterior staphyloma-associated macular hole in highly myopic eyes. Retina. 2006;26(1):25–31. | ||
Heimann H, Zou X, Jandeck C, et al. Primary vitrectomy for rhegmatogenous retinal detachment: an analysis of 512 cases. Graefes Arch Clin Exp Ophthalmol. 2006;244(1):69–78. | ||
Ruellan Y, Roussat B. [Obliteration of the posterior holes and tears of the retina by endodiathermy after vitrectomy]. J Fr Ophthalmol. 1984;7(12):807–812. French. | ||
Spaide R, Fisher Y. Removal of adherent cortical vitreous plaques without removing the internal limiting membrane in the repair of macular detachments in highly myopic eyes. Retina. 2005;25(3):290–295. | ||
Durairaj C, Chastain J, Kompella U. Intraocular distribution of melanin in human, monkey, rabbit, minipig and dog eyes. Exp Eye Res. 2012;98(1):23–27. | ||
Cheruvu NP, Amrite AC, Kompella UB. Effect of eye pigmentation on transscleral drug delivery. Invest Ophthalmol Vis Sci. 2008;49(1):333–341. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.