Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 10 » Issue 1
Profile of inhaled glycopyrronium bromide as monotherapy and in fixed-dose combination with indacaterol maleate for the treatment of COPD
Authors Prakash A, Babu KS, Morjaria JB
Received 14 May 2014
Accepted for publication 7 August 2014
Published 7 January 2015 Volume 2015:10(1) Pages 111—123
DOI https://doi.org/10.2147/COPD.S67758
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Anoop Prakash,1 K Suresh Babu,2 Jaymin B Morjaria1,3
1Department of Respiratory Medicine, Castle Hill Hospital, Cottingham, 2Department of Respiratory Medicine, Queen Alexandra Hospital, Cosham, Portsmouth, 3Department of Academic Respiratory Medicine, Hull York Medical School, University of Hull, Castle Hill Hospital, Cottingham, UK
Abstract: Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality. The cornerstone of pharmacological treatment for COPD is bronchodilation. Inhaled glycopyrronium bromide is a long-acting muscarinic antagonist developed as a maintenance treatment for patients with COPD. Phase III trials have shown that glycopyrronium produces rapid and sustained bronchodilation with an efficacy similar to tiotropium and is well tolerated, with a low incidence of muscarinic side effects in patients with moderate to severe COPD. A combination of glycopyrronium bromide with indacaterol maleate (QVA149) has recently been approved as a once-daily maintenance therapy in adult patients with COPD. Phase III trials (the IGNITE program) with QVA149 have demonstrated significant improvements in lung function versus placebo, glycopyrronium, and tiotropium in patients with moderate to severe COPD, with no safety concerns of note. Hence QVA149 is a safe treatment option for moderate to severe COPD patients in whom long-acting muscarinic antagonist monotherapy is inadequate.
Keywords: chronic obstructive pulmonary disease, glycopyrronium bromide, indacaterol maleate, umeclidinium, QVA149, long-acting muscarinic antagonist
Introduction
Chronic obstructive pulmonary disease (COPD) is a prevalent disease caused by chronic airway and pulmonary inflammation, primarily as a result of persistent inhalation of toxic particles and gases in tobacco smoke.1 The inflammatory process results in tissue repair, remodeling of the airways and lung parenchyma, and eventually loss of terminal and respiratory bronchioles followed by emphysematous destruction of the alveoli.2 Clinically this manifests as progressive airflow obstruction that is not fully reversible. The symptoms of COPD are well described, including both respiratory and systemic symptoms, with associated comorbidities, especially cardiovascular disease and lung cancer.1 Although components of the inflammatory pathway are therapeutic targets for ongoing drug development programs, the primary treatments for COPD do not address the underlying disease pathobiology, but rather the symptoms.3 Bronchodilation is the central treatment aim, and inhaled bronchodilators are the primary pharmacological intervention.4 Addition of an inhaled corticosteroid (ICS) is recommended as additional therapy for frequent exacerbations in more severe disease, although the evidence for improvement in lung function is questionable.5
Two classes of inhaled bronchodilators have been developed for COPD, ie, β2 adrenergic agonists and anticholinergics, which are available as short-acting and long-acting formulations. Short-acting bronchodilators are prescribed in milder disease, and have played a pivotal role in COPD management for 25 years; they are used on an “as needed” basis for breathlessness and exercise limitation.4 Long-acting β2 agonists (LABA) and anticholinergics play a central role in the maintenance treatment of COPD. The latter act as competitive antagonists at muscarinic receptors in the autonomic nervous system and exhibit a longer time-action profile (long-acting muscarinic antagonists, LAMA).4
Several distinct LAMAs with different characteristics and dosing schedules are approved for maintenance therapy of COPD, and a number are in clinical development. Tiotropium bromide (Spiriva®, Boehringer Ingelheim, Ingelheim, Germany) was the first once-daily LAMA approved for maintenance treatment in COPD and is extensively used in the management of COPD. In July 2012, aclidinium bromide (Eklira®, Almirall, Barcelona, Spain), a novel twice-daily LAMA, was approved in Europe and the USA, and more recently, in April 2014, umeclidinium bromide (Incruse® Ellipta®, GlaxoSmithKline, London, UK) was approved in Europe and the USA. The dry powder formulation of glycopyrronium bromide was developed for inhalation as a treatment for COPD under the compound code NVA237 (Seebri®, Novartis, Basel, Switzerland) and is delivered through the low-resistance Breezhaler® device.6 Glycopyrronium was approved in late 2012 as a once-daily maintenance treatment in adults with COPD and was the first once-daily alternative to tiotropium.
Subsequently, glycopyrronium was further developed with indacaterol maleate to form the first fixed-dose LABA-LAMA combination QVA149 (Ultibro®, Novartis). This was approved by the European Medicines Agency in late 2013 for the maintenance treatment of patients with moderate to severe COPD. Another LABA-LAMA fixed-dose combination was approved (umeclidinium-vilanterol, Anoro Ellipta, GlaxoSmithKline) by the US Food and Drug Administration and European Medicines Agency in December 2013 and May 2014, respectively, and others are in clinical development (eg, aclidinium-formoterol fumarate, Almirall).
The concept of dual bronchodilation with combination short-acting bronchodilators is well recognized, eg, the β2 agonist and antimuscarinic combination inhaler containing ipratropium bromide-albuterol (Combivent®), which has been in use for many years.7 For long-acting agents, a number of trials have assessed coprescribing of LABA and LAMA in separate inhalers.8 Generally, these trials, conducted using tiotropium coprescribed with a number of LABAs, consistently found a greater effect on lung function with the combination than with single agents, with some benefit in patient-reported outcomes and no safety concerns apparent.8–10
An important issue for both regulators and practicing clinicians is to understand any benefit of coadministration of a LAMA-LABA as the fixed-dose combination over LAMA monotherapy. Further, some centers in the UK have revised their prescribing practice and placed LABA-LAMA coprescribing ahead of combination LABA-ICS fixed-dose combinations. In this review, we attempt to address these issues and report and interpret the Phase III trial data available for glycopyrronium and QVA149 in both placebo-controlled and active-controlled studies for the treatment of COPD.
Relevant literature searches were conducted in PubMed to identify Phase III studies that compared glycopyrronium with placebo, tiotropium, and QVA149. Abstracts presented at international respiratory conferences, ie, the American Thoracic Society (2008–2013) and the European Respiratory Society (2008–2013), were searched, as was the website of the manufacturer/distributor of inhaled glycopyrronium (Novartis).
Pharmacology and pharmacokinetics
Glycopyrronium (3-(2-cyclopentyl-2-hydroxy-2-phenylacetyloxy)-1,1-dimethyl-pyrrolidinium bromide), like other muscarinic antagonists, ie, ipratropium, tiotropium, and aclidinium bromide, is a synthetic quaternary ammonium congener of atropine with a distinct molecular structure (Figure 1).11 Quarternization of the tertiary amino function minimizes oral bioavailability and blood–brain barrier permeability, and hence reduces systemic side effects if swallowed.12
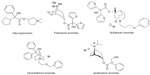  | Figure 1 Inhaled, long-acting muscarinic antagonists, showing the molecular structure of glycopyrronium bromide compared with other anticholinergics. |
Mechanism of action of muscarinic antagonists
Parasympathetic nerves are the predominant neural pathways in the airways and exert bronchoconstrictor, mucus secretory, and other localized effects via release of acetylcholine that acts on multiple muscarinic receptors.13 The effects are regulated by G protein-coupled muscarinic M1 and M3 receptors as well as M2 receptors. M1 receptors have been located in the bronchus, fibroblasts, bronchial epithelial cells, and parasympathetic ganglia. M2 receptors preferentially couple to the G protein Gxo/i and function to counteract the β2 adrenoreceptor-mediated relaxant pathway by antagonizing the synthesis and accumulation of cyclic adenosine monophosphate (cAMP).14 These channels are coupled to β2 receptors via the Gs signaling protein coupled to the Gi signaling protein in the airway smooth muscle. This interaction may result in airway smooth muscle contraction via reversal of hyperpolarization mediated by the β2 receptor and maxi-K+ channels. M2 receptors are located on presynaptic parasympathetic nerve endings at the neuromuscular junction with feedback inhibition of acetylcholine release and influence bronchial smooth muscle to counteract bronchodilation by inhibiting β2 receptor-mediated cAMP production. The M3 receptor preferentially couples to the heterotrimeric G protein, Gq11. This results in stimulation of phospholipase C and an elevation in intracellular calcium. The M3 receptor is the primary muscarinic receptor mediating bronchial and tracheal smooth muscle contraction despite its markedly lower expression when compared with M2 receptors. Moreover, M3 receptors are implicated in mucus secretion as well as vasodilatation in vascular smooth muscle cells via diffusion of nitric oxide synthesized in vascular endothelial cells.
Mechanism of action of glycopyrronium
Glycopyrronium acts as a highly potent, competitive muscarinic receptor antagonist that binds to muscarinic receptors in bronchial smooth muscle and inhibits acetylcholine-mediated bronchoconstriction. Glycopyrronium binds with high affinity to M1–3 receptors. It exhibits higher selectivity (4–5-fold) for M1 and M3 subtypes over M2, and shows faster dissociation from M2 than from M1 and M315 Tiotropium shares similar characteristics, including a higher selectivity for M3 receptors than for M2 receptors, and tiotropium and aclidinium also dissociate more slowly from the M3 receptor than from the M2 receptor. There are some subtle differences when compared with tiotropium, shown in in vitro comparisons during the drug development process. Glycopyrronium exhibits greater binding selectivity for M3 over M2 receptors than tiotropium, and has higher kinetic selectivity and faster dissociation from M2 receptors than from M3 receptors when compared with tiotropium.15
Mechanism of action of QVA149
The molecular structure and mode and speed of action of indacaterol have been reviewed in detail previously.16 Briefly, indacaterol is a long-acting β2 adrenoceptor partial agonist with high intrinsic activity that stimulates intracellular adenylyl cyclase, which converts adenosine triphosphate to cAMP.16 The resulting increased intracellular cAMP levels lead to relaxation of bronchial smooth muscle.
The pharmacological basis for any synergistic effect of the codelivery of LAMA and LABA is not fully characterized. Generally, it is hypothesized from in vivo and in vitro observations that interactions between adrenergic and cholinergic pathways can be expected, including modulation of cholinergic neurotransmission by β2 agonists,17 and may potentially lead to synergistic effects.18 Laboratory studies suggest that synergistic effects may occur via a variety of mechanisms: “cross talk” between β2 adrenoceptors and muscarinic receptors that influences β2 agonist-induced relaxation via activation of protein kinase C;19 and β2 agonists may indirectly release acetylcholine and amplify LAMA-induced bronchial smooth muscle relaxation.20
The pharmacological profile and nonclinical evaluation of QVA149 is based essentially on data available for its individual components. A study reported in the European Medicines Agency regulatory submission employed carbachol as the contractile stimulus, and the relaxant effect induced by QVA149 was reported to be equivalent to the additive relaxant effect of indacaterol and glycopyrronium applied individually.21
Dosage, administration, and absorption
Each dry powder capsule contains 63 μg of glycopyrronium bromide, which is equivalent to 50 μg of glycopyrronium. Administered via the Breezhaler device,5 each delivered dose (ie, the amount of drug that leaves the inhaler) contains 55 μg of glycopyrronium bromide, which is equivalent to 44 μg of glycopyrronium.22 Glycopyrronium given via the Breezhaler is rapidly absorbed, reaching peak plasma levels at 5 minutes post-dose,15 with an absolute bioavailability of about 45% of the delivered dose. About 90% of systemic exposure following inhalation is due to lung absorption and 10% is due to gastrointestinal absorption.23
The dry powder capsule formulation of QVA149 was developed based on the glycopyrronium capsule; the amount of glycopyrronium is unchanged (63 μg of glycopyrronium bromide, which is equivalent to 50 μg of glycopyrronium). Combining the two bronchodilators in QVA149 results in an altered fine particle mass and fine particle fraction of indacaterol when compared with the profile of indacaterol monotherapy, and hence a dose reduction of the indacaterol component from 150 μg to 110 μg.21 When administered via the Breezhaler, the delivered dose (the dose that leaves the mouthpiece of the inhaler) contains 110 μg of indacaterol maleate equivalent to 85 μg of indacaterol and 54 μg of glycopyrronium bromide equivalent to 43 μg of glycopyrronium.21 Following inhalation of QVA149, the median times to peak plasma concentration of indacaterol and glycopyrronium are approximately 15 minutes and 5 minutes, respectively.22 No differences in absorption, bioavailability, tissue distribution, and metabolism of glycopyrronium and indacaterol have been reported with either monotherapy or when combined in QVA149.21
Clinical trial programs of glycopyrronium bromide and QVA149
The efficacy and safety profile of inhaled glycopyrronium (50 μg once daily) in patients with moderate to severe COPD was established in the GLOW (GLycopyrronium bromide in chronic Obstructive pulmonary disease airWays) program, which consisted of six Phase III randomized trials. GLOW-124 and GLOW-225 were the “pivotal” Phase III registration-track efficacy and safety studies and were of 26 weeks and 52 weeks in duration, respectively, and compared glycopyrronium with placebo (both studies) and open-label tiotropium (GLOW-2) delivered by the Handihaler® device. These studies have been subject to a post hoc pooled analysis.26 GLOW-3 was a 3-week, placebo-controlled crossover trial that evaluated the efficacy of glycopyrronium in improving exercise tolerance.27 GLOW-4 assessed the long-term safety of open-label once-daily tiotropium versus glycopyrronium in Japanese patients with moderate to severe COPD.28 GLOW-5 compared the efficacy and safety of glycopyrronium against blinded tiotropium over 12 weeks.29 GLOW-6 was a double-blind, 12-week study comparing once-daily dual bronchodilation by coadministration of glycopyrronium and indacaterol (150 μg) with monotherapy comprising indacaterol (150 μg) in patients with moderate to severe COPD.30 The primary and secondary outcomes assessed in these studies are summarized in Table 1.24–30
The clinical development program for QVA149, known by the acronym IGNITE (Indacaterol and GlycopyrroNium bromide clInical sTudiEs), comprises eleven randomized trials ranging in duration from 6 to 64 weeks that have enrolled more than 10,000 patients. Seven studies have been completed and published. The principal investigations of efficacy were the 26-week ILLUMINATE31 and SHINE trials,32 primarily focusing on lung function, and the 64-week SPARK33 trial that assessed efficacy with respect to exacerbation frequency. Dyspnea and exercise tolerance were assessed in the 3-week BLAZE34 and 6-week BRIGHT35 trials, respectively. ENLIGHTEN36 was a 52-week safety trial, and the BEACON37 trial was a noninferiority study of bronchodilation at 4 weeks compared with coadministration of the monocomponents. Five trials from the IGNITE program have been subject to meta-analysis.38 Details of these trials are provided in Table 2.
Therapeutic efficacy of glycopyrronium: comparison with placebo
Lung function
In GLOW-1 and GLOW-2, treatment with glycopyrronium was associated with superior trough forced expiratory volume in 1 second (FEV1) at 12 weeks (primary endpoint) compared with placebo (Δ=108 mL and Δ=97 mL, respectively; P<0.001).24,25 In the pooled analysis, the difference at week 26 was 125 mL. The findings in SHINE were consistent, with glycopyrronium at week 26 improving FEV1 by a similar magnitude over placebo (Δ=120 mL, P<0.001).25
Dyspnea
Breathlessness was assessed by the Transition Dyspnea Index (TDI) focal score (the lower the score, the more deterioration in severity of dyspnea). From baseline to week 26, TDI was significantly greater with glycopyrronium than with placebo in GLOW-1, GLOW-2, and SHINE (Table 3).24,25,32 The mean difference reached the between-group difference threshold for a minimum clinically important difference (MCID) of at least one point in GLOW-1. In responder analyses, significantly more patients receiving glycopyrronium compared with placebo achieved an MCID improvement in TDI focal score from baseline to end of study in the GLOW-1, GLOW-2, and SHINE studies (Table 3).
Health status
Glycopyrronium was associated with a significantly lower St George’s Respiratory Questionnaire (SGRQ) score than placebo after 26 weeks of treatment in GLOW-1 (Δ= −2.81, P<0.01), but this between-group difference did not reach the threshold for a mean MCID of at least 4 points.24 Significantly more patients treated with glycopyrronium reached the MCID than those on placebo (Table 3). In GLOW-2, the decrease in SGRQ scores from baseline to weeks 12, 26, and 52 were consistently superior in glycopyrronium-treated patients compared with placebo, but again did not reach the MCID (Δ=−3.17, −3.38, −3.32, respectively; P<0.001).25 In SHINE, glycopyrronium was not superior to placebo at week 26, and the responder analysis showed the proportion reaching the MCID was not significantly different from placebo.32
Exacerbations
In GLOW-1 and GLOW-2, glycopyrronium significantly reduced the time to first moderate (requiring treatment with systemic corticosteroids or antibiotics) to severe (requiring hospital admission or emergency treatment) COPD exacerbation by 31% over 26 weeks (hazard ratio 0.69, 95% confidence interval [CI] 0.500–0.949) and 34% over 52 weeks (hazard ratio 0.66, 95% CI 0.520–0.850) versus placebo.24,25
Rescue medication
Glycopyrronium was associated with significantly less use of rescue medication than placebo in GLOW-1 and GLOW-2. However, the difference compared with placebo was not significant in SHINE (Table 3).24,25
Lung volumes and exercise capacity
Across GLOW-1 and GLOW-2, glycopyrronium resulted in a significantly higher inspiratory capacity than placebo at all assessed time points up to week 52.24,25 In GLOW-3, glycopyrronium resulted in superior mean exercise endurance time (primary endpoint) than placebo at day 21 (Δ=88.9 seconds, P<0.001).27 Glycopyrronium compared with placebo reduced lung hyperinflation as assessed by the inspiratory capacity at isotime during a constant-load cycle ergometry test which was significantly higher than placebo (day 21, 2.22 L versus 2.02 L, P<0.001).
Therapeutic efficacy of glycopyrronium: comparison with tiotropium and indacaterol
Lung function
In GLOW-2 and GLOW-5, generally there were no differences between glycopyrronium and tiotropium in values for key spirometry-assessed parameters, except for a faster onset of action in favor of glycopyrronium.25,29 In SHINE, there was no difference in efficacy between glycopyrronium, tiotropium, and indacaterol, with the improvement in trough FEV1 over placebo at week 26 (primary endpoint) being of similar magnitude (Table 3) and in peak FEV1 values over placebo (Δ=250 mL, Δ=240 mL, and Δ=230 mL, respectively; all P<0.001).32
Dyspnea
GLOW-2 reported no significant differences in TDI focal scores from baseline to week 12, 26, or 52 between glycopyrronium and tiotropium (Table 3).25 GLOW-5 findings were comparable, with no difference in improvements in TDI focal scores between glycopyrronium and blinded tiotropium at week 12 (Δ= −0.188; not statistically significant).29 In SHINE, statistically significant improvements in TDI scores versus placebo were observed with glycopyrronium and indacaterol at weeks 12 and 26.32 A significantly greater proportion of patients treated with glycopyrronium or indacaterol achieved the MCID for TDI at week 26 than placebo; this was not the case for tiotropium (Table 3).
Health status
Both glycopyrronium and tiotropium were superior to placebo in SGRQ total score at weeks 12, 26, and 52, with no difference between glycopyrronium and tiotropium (Table 3).24,25 GLOW-5 also reported a comparable SGRQ total score between glycopyrronium and tiotropium at week 12, with a treatment difference between glycopyrronium and tiotropium of 0.65 (not statistically significant; Table 3).29 In the SHINE study, the difference from placebo at week 26 was not different with indacaterol and tiotropium, and the proportion reaching the MCID was not different from placebo.32
Exacerbations
In GLOW-2, glycopyrronium (34%) and tiotropium (39%) were comparable, and both were superior to placebo in reducing the risk of exacerbations in terms of time to first moderate or severe exacerbation per year (Table 3).25 Similarly, in GLOW-5, no significant treatment difference was observed between glycopyrronium and tiotropium with respect to number of moderate or severe COPD exacerbations per year.29 In SHINE, the number of patients experiencing an exacerbation was similar with glycopyrronium, tiotropium, and indacaterol (Table 3).32
Rescue medication
The change from baseline in mean daily number of puffs of rescue medication was not different between glycopyrronium and tiotropium.25,29 The SHINE study showed less rescue medication use with indacaterol than with glycopyrronium (−0.65 versus −0.30, P=0.011).32
Lung volumes and exercise capacity
In GLOW-2, inspiratory capacity was comparable with that in the tiotropium group at almost all evaluated time points on day 1 and at weeks 12 and 52.25 In GLOW-5, the inspiratory capacity was significantly higher with glycopyrronium versus tiotropium at 30 minutes and 2 hours post-dose on day 1 (Δ=0.078 L, P<0.001; Δ=0.098 L, P<0.001, respectively). Inspiratory capacity 24 hours post-dose at week 12 was similar in both treatment groups (change from baseline 0.126 L versus 0.148 L for glycopyrronium and tiotropium, respectively, not statistically significant).29
Safety profile of glycopyrronium as monotherapy
In GLOW-1, there were no safety concerns identified with the use of glycopyrronium when compared with placebo. The mean QTc interval did not differ significantly between glycopyrronium and placebo, although it should be noted that a prolonged interval (QTc >450 ms for males and >470 ms for females) was an exclusion criterion.24 GLOW-2 confirmed the acceptable safety profile of glycopyrronium and showed it to be similar to that of open-label tiotropium (Table 4).25 Rates of adverse events were similar between the three study arms. Anti-muscarinic side effects, such as dry mouth, occurred at a low frequency in the three study arms. No deaths reported in GLOW-2 were considered to be related to the study medication. GLOW-3 found glycopyrronium to have a good safety profile, ie, adverse events (29.4%), broadly similar to that of placebo (24.5%). Most adverse events were mild to moderate in severity and considered unrelated to the study medication.27
GLOW-4 showed a similar overall incidence of adverse events between glycopyrronium and tiotropium.28 No significant changes in heart rate or prolongation of the QTcF were noted over the 52-week study period. The incidence of dry mouth was less frequent with glycopyrronium (1.6%) than with tiotropium (5%). As in GLOW-2 and GLOW-4, the overall incidences of adverse events in GLOW-5 were similar in the glycopyrronium (40.4%) and tiotropium (40.6%) groups. The most frequently reported adverse event was worsening of COPD, seen with a higher frequency in the tiotropium group (17.6%) when compared with the glycopyrronium group (15.3%). Other more frequently occurring adverse events were nasopharyngitis, headache, upper respiratory tract infection, and urinary tract infection, which occurred more frequently in the glycopyrronium group than in the tiotropium group.29 Adverse events leading to discontinuation occurred in a comparable number of patients in both groups.
Therapeutic efficacy of QVA149: comparison with indacaterol, glycopyrronium, and tiotropium
Lung function
The 26-week SHINE study was designed to determine if QVA149 met the “combination rule”, ie, that it was superior to its monocomponents, indacaterol and glycopyrronium; in addition, the study had placebo and active-control (open-label tiotropium once daily) arms.32 Trough FEV1 at week 26 (primary efficacy endpoint) was significantly improved with QVA149 compared with the monocomponents indacaterol, glycopyrronium, and tiotropium (Table 4 and Figure 2A). Moreover, at week 26, peak FEV1 at 2 hours post-dose was superior for QVA149; with treatment differences of 170 mL, 150 mL, and 160 mL versus indacaterol, glycopyrronium, and tiotropium, respectively (P<0.001 for all treatment comparisons). In a meta-analysis across five trials (4,842 patients), the glycopyrronium-indacaterol combination group showed a significant increase in trough FEV1 (70 mL; P<0.0001) when compared with the tiotropium group and glycopyrronium group.38 This further confirms that QVA149 is superior to LAMA monotherapy.
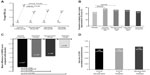  | Figure 2 Effect of QVA149, its monocomponents, and tiotropium on mean trough FEV1 (A), responder analysis for improvement in TDI score (B), SGRQ score (C), and annualized rate ratio of moderate or severe COPD exacerbations (D). |
Dyspnea
The primary objective of BLAZE was to demonstrate the superiority of QVA149 over placebo in improvement of patient-reported dyspnea, as assessed by a novel, self-administered Basal Dyspnea Index/TDI score after 6 weeks, with superiority over tiotropium as a key secondary objective.34 BLAZE found that, after 6 weeks, the TDI total score was significantly improved with QVA149 and achieved the MCID versus placebo, and was superior to tiotropium (Table 4). As a secondary endpoint, SHINE showed that the TDI focal score was significantly improved with QVA149 at week 26 and achieved the MCID versus placebo, and was superior to tiotropium (Figure 2B).32
The meta-analysis by Rodrigo et al reported that the combination of QVA149 compared with tiotropium was associated with a 19% greater likelihood of a MCID in TDI, with a number needed to treat for benefit of 11.38
Health status
In the SHINE study, QVA149 significantly improved SGRQ scores at week 26 compared with placebo and tiotropium, but with no significant difference versus indacaterol and glycopyrronium (Table 4 and Figure 2C).32 Moreover, more patients receiving QVA149 achieved the MCID of 4 compared with those receiving tiotropium, although no marked differences were noted versus indacaterol and glycopyrronium. Also, the proportion of patients experiencing major improvements in these scores (TDI ≥3 points and SGRQ >8 points) was also significantly higher with QVA149, over tiotropium for the former and over tiotropium and glycopyrronium for the latter. In the SPARK study, although the responder analyses with SGRQ for MCID were not significant, substantial improvement (>8 points on the SGRQ scale) was significantly higher for QVA149 than glycopyrronium (46.0% versus 38.7%; P=0.013) and tiotropium (46.0% versus 37.3%; P=0.006).32
Moreover, patients administered QVA149 compared with tiotropium were reported to be 16% more likely to achieve an MCID assessed by the SGRQ, with a number needed to treat for benefit of 11.38 Similarly, a significant increase was found in the number of patients achieving an MCID on the SGRQ in favor of QVA149 compared with glycopyrronium monotherapy, with a number needed to treat of 12.38
Exacerbations
SPARK was a 64-week, randomized trial of QVA149 compared with glycopyrronium (double-blind), or open-label tiotropium.33 The primary objective was to demonstrate superiority of QVA149 compared with glycopyrronium for the rate of moderate or severe COPD exacerbations during the treatment period and over tiotropium as a secondary objective. Exacerbations were defined as mild if self-managed by the patient, moderate when requiring treatment with systemic corticosteroids or antibiotics or both, or severe when requiring hospital admission or emergency treatment. QVA149 significantly reduced the annualized rate of moderate to severe exacerbations versus glycopyrronium by 12% (0.84 [95% CI 0.75–0.94] versus 0.95 [CI 0.85–1.06]; 0.88 [95% CI 0.77–0.99]; P=0.038, Figure 2D). The 10% reduction compared with tiotropium was not statistically significant.
Lung volumes and exercise capacity
BRIGHT was a 3-week, randomized trial that evaluated QVA149 versus placebo and tiotropium on exercise tolerance, hyperinflation, lung function, and volumes in patients with moderate to severe COPD.35 At day 21, QVA149 significantly improved inspiratory capacity during exercise (at isotime), compared with both placebo (Δ=0.32 L, P<0.001) and tiotropium (Δ=0.13 L, P<0.01), and with significant improvements in trough inspiratory capacity of 0.19 L (P<0.01) and 0.14 L (P<0.01), respectively. QVA149 improved exercise endurance time (by 59.5 seconds) compared with placebo (P=0.006) but not compared with tiotropium.
Therapeutic efficacy of QVA149: comparison with salmeterol-fluticasone
ILLUMINATE was a 26-week randomized trial that compared QVA149 with twice-daily salmeterol-fluticasone 50/500 μg (administered via the Accuhaler® device) in exacerbation-free (no history in the previous year) patients with moderate to severe COPD.31 QVA149 was associated with a significantly higher FEV1 AUC0–12 h than salmeterol-fluticasone (Δ=138 mL, P<0.0001). There was a significant increase in TDI focal score at weeks 12 and 26 with QVA149 (Table 4). At week 26, significantly more patients treated with QVA149 achieved the one-point MCID in TDI. QVA149 patients used significantly less rescue medication and daytime rescue medication (Δ=−0.32, 95% CI −0.52 to −0.13; P=0.0013) versus patients receiving salmeterol-fluticasone. SGRQ total scores improved in both arms, with no between-group differences, but there were significant improvements in breathlessness scores favoring QVA149.
Safety profile of glycopyrronium when combined with indacaterol (QVA149)
Across the seven Phase III indacaterol (QVA149) trials, the safety profile was shown to be similar to that of placebo and the component LAMA and LABA monotherapies.31–37 A pooled 6-month safety analysis was performed from three placebo-controlled and active-controlled Phase III studies (SHINE, ILLUMINATE, ENLIGHTEN), also including interim 6-month data from ARISE, to give a total population of 3,153 patients with moderate to severe COPD.39 There was no evidence of any increased risk of cardiocerebral vascular events (cardiac arrhythmias and QTcF prolongation) with QVA149 versus all comparators. A similar lack of increased serious and major adverse events have been reported in a meta-analysis.40
Dual bronchodilation: essential considerations and perspectives
Direct comparisons between treatments are often not available in clinical medicine. Because of the requirements of the regulators, the relatively large clinical trial programs for glycopyrronium bromide and QVA149 included head-to-head comparisons that provide useful comparative data for once-daily mono and dual bronchodilation. This unique data set allows certain conclusions to be drawn with respect to treatment algorithms in COPD and raises interesting issues that need addressing in subsequent studies.
The comparison of lung function with QVA149 and glycopyrronium found that the effect of dual bronchodilation was not synergistic, with the improvements in FEV1 actually being less than additive. This conclusion is somewhat inconsistent with earlier8 albeit limited trials of coprescribing of the monotherapies, which had suggested that there may be more than an additive effect, and that any pharmacological interaction between the different receptor-activated pathways is limited.
Despite the lack of synergy, the improvement in bronchodilation supports the current Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendation of using LABA-LAMA dual bronchodilation as a treatment option in GOLD B, C, and D stages. It also suggests that the current National Institute for Health and Care Excellence guidelines, which recommend a step up from monotherapy to dual bronchodilation only when the initial treatment was a LABA, and then if ICS are declined or not tolerated, are now due for revision. However, in relation to patient experience and patient-reported outcomes, and to exacerbations, the findings of the individual trials suggest the superiority of QVA149 is not so clear, and raise questions not only about the impact dual bronchodilation has in terms of clinically relevant endpoints, but also about the concept of MCID and whether the measure of MCID for patient-reported outcomes remains valid when the comparator is an active treatment rather than placebo. Indeed, given the view that the beneficial effect of bronchodilators is “intrinsically limited by the nature of the disease”,18 the expectation is that “the mean magnitude of differences between one and two bronchodilators cannot be huge”.18
However, another important consideration of benefit is the view that, in a polyphenotypic disease such as COPD, it is preferable to consider individual patient response through responder analyses, ie, providing the proportion of patients in which the treatment effect reached the MCID rather than providing an average treatment effect. This approach is commonly used in other chronic diseases that have different phenotypic expressions, eg, type 2 diabetes.
Finally, the IGNITE trial program offers insights into the relative efficacy of QVA149 against the guideline-recommended combination LABA-ICS. An immediately obvious limitation of the ILLUMINATE trial is that the patients selected were exacerbation-free in the year before trial entry. This research found that in this selected population, which included mainly patients (80%) with moderate COPD, QVA149 resulted in greater improvements in lung function, as well as in dyspnea and rescue medication use, but not in health-related quality of life. These results are encouraging, but further studies are required in patients with severe COPD and a recent history of exacerbations.
Conclusion
Optimized bronchodilation is an important treatment goal in COPD management, and a number of new anticholinergics have been approved in the last year. The approval of QVA149, a fixed-dose combination of glycopyrronium and indacaterol, has provided the first insights into the potential benefits of combining a LABA and a LAMA in the same inhaler. The rationale for combining these drugs was clear and, coupled with once-daily dosing of two drugs in a single inhaler with a fast onset of effect, suggested the potential for further benefit in terms of patient adherence. Reviewing the totality of evidence for glycopyrronium and QVA149 Phase III studies shows that there are no major clinical differences between glycopyrronium and tiotropium and that QVA149 provides superior lung function improvement over LAMA monotherapy, but the extent of the improvement in lung function is more limited than expected and no synergy was shown. There were no additional safety issues with QVA149 over its monocomponents. The data to date firmly suggest a place for QVA149 as an important treatment option in patients with moderate to severe COPD that is not adequately controlled by LAMA monotherapy before escalation to LABA-ICS. Moreover, with the licensing and late-stage assessment of other dual bronchodilators, patients will not only have options available for inhaler devices but also flexibility in the dosing regimen.
Author contributions
AP and JBM conceived the review and its content, including all interpretation of the studies described, based on discussions in September 2013. KSB reviewed and commented on the first draft and approved the final version. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
Aidan McManus and Ana Martins-Kaczor from Edge Medical Communications assisted JBM to write a first draft of the manuscript and provided subsequent editorial support as directed by JBM. This editorial assistance was funded by Novartis UK, which reviewed the manuscript for medical accuracy but not content. KSB has received honoraria for speaking and financial support to attend meetings held by Wyeth, Chiesi, and AstraZeneca. JBM has received honoraria for speaking and financial support to attend meetings/advisory boards organized by Wyeth, Chiesi, Pfizer, MSD, Boehringer Ingelheim, Teva, GSK/Allen and Hanburys, Napp, Almirall, AstraZeneca, and Novartis. AP reports no conflicts of interest in this work.
References
Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. | |
Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest. 2013;143(5):1436–1443. | |
Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12(7):543–559. | |
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | |
Babu KS, Kastelik JA, Morjaria JB. Inhaled cortiocsteroids in COPD: a pro-con perspective. Br J Clin Pharm. 2014;78(2):282–300. | |
Pavkov R, Mueller S, Fiebich K, et al. Characteristics of a capsule based dry powder inhaler for the delivery of indacaterol. Curr Med Res Opin. 2010;26(11):2527–2533. | |
Combivent® (ipratropium bromide and albuterol sulfate) Inhalation Aerosol Prescribing Information. Boehringer Ingelheim, 2012. Available from: http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Combivent+Respimat/CMVTRSPT.pdf. Accessed January 31, 2014. | |
Tashkin DP, Ferguson GT. Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir Res. 2013;14:49. | |
van Noord JA, Aumann JL, Janssens E, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129(3):509–517. | |
van Noord JA, Aumann JL, Janssens E, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J. 2005;26(2):214–222. | |
Sechaud R, Renard D, Zhang-Auberson L, Motte Sde L, Drollmann A, Kaiser G. Pharmacokinetics of multiple inhaled NVA237 doses in patients with chronic obstructive pulmonary disease (COPD). Int J Clin Pharmacol Ther. 2012;50(2):118–128. | |
Ali-Melkkila T, Kanto J, Iisalo E. Pharmacokinetics and related pharmacodynamics of anticholinergic drugs. Acta Anaesthesiol Scand. 1993;37(7):633–642. | |
Barnes PJ. Muscarinic receptor subtypes in airways. Life Sci. 1993; 52(5–6):521–527. | |
Sykes DA, Dowling MR, Charlton SJ. Exploring the mechanism of agonist efficacy: a relationship between efficacy and agonist dissociation rate at the muscarinic M3 receptor. Mol Pharmacol. 2009;76(3):543–551. | |
Sykes DA, Dowling MR, Leighton-Davies J, et al. The influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropium. J Pharmacol Exp Ther. 2012;343(2):520–528. | |
Moen MD. Indacaterol: in chronic obstructive pulmonary disease. Drugs. 2010;70(17):2269–2280. | |
Cazzola M, Molimard M. The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23(4):257–267. | |
Roche N, Chanez P. Bronchodilator combinations for COPD: real hopes or a new Pandora’s box? Eur Respir J. 2013;42(6):1441–1445. | |
Cazzola M, Segreti A, Matera MG. New developments in the combination treatment of COPD: focus on umeclidinium/vilanterol. Drug Des Devel Ther. 2013;7:1201–1208. | |
Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450–504. | |
European Medicines Agency. Ultibro Breezhaler: European Medicines Agency assessment report. 2013. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002679/WC500151257.pdf. Accessed December 8, 2013. | |
Novartis Europharm Ltd. Seebri Breezhaler (glycopyrronium bromide) powder for inhalation: EU summary of product characteristics. 2012. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002430/WC500133769.pdf. Accessed December 8, 2013. | |
Sechaud R, Sudershan M, Perry S, et al. Efficient deposition and sustained lung concentrations of NVA237 after inhalation via the Breezhaler device in man. Eur Respir J. 2012;40 Suppl 56:P4839. | |
D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156. | |
Kerwin E, Hebert J, Gallagher N, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012;40(5):1106–1114. | |
D’Urzo A, Kerwin E, Overend T, D’Andrea P, Chen H, Goyal P. Once daily glycopyrronium for the treatment of COPD: pooled analysis of the GLOW1 and GLOW2 studies. Curr Med Res Opin. 2014;30(3):493–508. | |
Beeh KM, Singh D, Di Scala L, Drollmann A. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulmon Dis. 2012;7:503–513. | |
Sekiya M, Kawayama T, Fukuchi Y, et al. Safety and efficacy of NVA237 once daily in Japanese patients: the GLOW4 trial. Eur Respir J. 2012;40 Suppl 56:P2103. | |
Chapman KR, Beeh KM, Beier J, et al. A blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 study. BMC Pulm Med. 2014;14(1):4. | |
Walter V, Goyal P, Jack D, Chen H, Henley M, Mcbryan D. Co-administration of glycopyrronium and indacaterol improves lung function and symptoms in patients with COPD versus indacaterol alone: the GLOW6 study. Thorax. 2013;68:A181–A182:P232. | |
Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE):a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60. | |
Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494. | |
Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK):a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. | |
Mahler D, Decramer M, D’Urzo A, et al. Superior lung function with once-daily QVA149 translates into improvements in patient reported breathlessness compared with placebo and tiotropium in COPD patients: the BLAZE study. Am J Respir Crit Care Med. 2013;187:A6070. | |
Beeh KM, Korn S, Beier J, et al. QVA149 once daily improves exercise tolerance and lung function in patients with COPD: the BRIGHT study. Thorax. 2012;67 Suppl 2:P191. | |
Dahl R, Chapman KR, Rudolf M, et al. Safety and efficacy of dual bronchodilation with QVA149 in COPD patients: the ENLIGHTEN study. Respir Med. 2013;107(10):1558–1567. | |
Dahl R, Jadayel D, Alagappan VK, Chen H, Banerji D. Efficacy and safety of QVA149 compared to the concurrent administration of its monocomponents indacaterol and glycopyrronium: the BEACON study. Int J Chron Obstruct Pulmon Dis. 2013;8:501–508. | |
Rodrigo GJ, Plaza V. Efficacy and safety of a fixed-dose combination of indacaterol and glycopyrronium (Qva149) for the treatment of COPD: a systematic review. Chest. 2014;146(2):309–317. | |
Welte T, Vogelmeier L, Dahl R, et al. Once-daily QVA149 has a good safety profile in patients with COPD. Eur Respir J. 2013;42 Suppl 57:P757. | |
Lu Y, Chen H, D’Andrea P, Banerji D. Once-daily QVA149 shows no increase in the risk of cardio- and cerebro-vascular events, pneumonia and exacerbation events, and mortality compared with placebo: a network meta-analysis across multiple safety databases. Am J Respir Crit Care Med. 2013;187:A1486. | |
Singh D. New combination bronchodilators for COPD: current evidence and future perspectives. British Journal of Clinical Pharmacology. 2014. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




