Back to Journals » International Journal of Women's Health » Volume 6
Prescribing menopausal hormone therapy: an evidence-based approach
Authors Sood R , Faubion S, Kuhle C, Thielen J, Shuster L
Received 23 August 2013
Accepted for publication 3 October 2013
Published 11 January 2014 Volume 2014:6 Pages 47—57
DOI https://doi.org/10.2147/IJWH.S38342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Richa Sood, Stephanie S Faubion, Carol S Kuhle, Jacqueline M Thielen, Lynne T Shuster
Division of General Internal Medicine, Women's Health Clinic, Mayo Clinic, Rochester, MN, USA
Abstract: The constantly changing landscape regarding menopausal hormone therapy (MHT) has been challenging for providers caring for menopausal women. After a decade of fear and uncertainty regarding MHT, reanalysis of the Women's Health Initiative data and the results of recent studies have provided some clarity regarding the balance of risks and benefits of systemic MHT. Age and years since menopause are now known to be important variables affecting the benefit-risk profile. For symptomatic menopausal women who are under 60 years of age or within 10 years of menopause, the benefits of MHT generally outweigh the risks. Systemic MHT initiated early in menopause appears to slow the progression of atherosclerotic disease, thereby reducing the risk of cardiovascular disease and mortality. During this window of opportunity, MHT might also provide protection against cognitive decline. In older women and women more than 10 years past menopause, the risk-benefit balance of MHT is less favorable, particularly with regard to cardiovascular risk and cognitive impairment. For women entering menopause prematurely (<40 years), MHT ameliorates the risk of cardiovascular disease, osteoporosis, and cognitive decline. Nonoral administration of estrogen offers advantages due to the lack of first-pass hepatic metabolism, which in turn avoids the increased hepatic synthesis of clotting proteins, C-reactive protein, triglycerides, and sex hormone-binding globulin. The duration of combined MHT use is ideally limited to less than 5 years because of the known increase in breast cancer risk after 3–5 years of use. Limitations to use of estrogen only MHT are less clear, since breast cancer risk does not appear to increase with use of estrogen alone. For women under the age of 60 years, or within 10 years of onset of natural menopause, MHT for the treatment of bothersome menopausal symptoms poses low risk and is an acceptable option, particularly when nonhormonal management approaches fail.
Keywords: hormone therapy, hot flash, flush, menopause
Introduction
Systemic estrogen containing menopausal hormone therapy (MHT) has been available for over half a century to provide relief for hot flashes, night sweats, and symptoms of urogenital atrophy. Multiple observational studies have reported protective effects of MHT in terms of cardiovascular disease and mortality.1 This led to the inception of randomized clinical trials designed to test the hypothesis that MHT could indeed promote cardiovascular health and protect against conditions of aging, while also treating menopausal symptoms. The results from these trials, notably the Women’s Health Initiative (WHI) and Heart and Estrogen/Progestin Replacement Study, were contrary to what was expected, ie, they reported an unfavorable balance of risks compared with benefits for women given hormone therapy compared with placebo. As a result, there was a major shift in perspective on MHT use over the last decade that led to a significant decline in prescriptions of MHT.2 Since then, data from the WHI have been reanalyzed, several conclusions revised, and newer data from clinical trials including the Kronos Early Estrogen Prevention Study and Danish Osteoporosis Prevention Study have become available. The timing hypothesis and concept of a “window of opportunity”3 have received greater attention, in recognition that the risks and benefits of hormones vary based on a woman’s age and time since menopause, with the effects of MHT being primarily beneficial when initiated in younger women closer to the onset of menopause, but harmful when initiated later in life or further from the onset of menopause.
Updated guidelines for MHT use have been provided by the North American Menopause Society, European Menopause Society, British Menopause Society, and multiple others, with consensus statements incorporating this concept issued in 2012 and 2013.4–6 In this review, our intent is to provide evidence-based, practical tips to clinicians faced with the decision of whether or not to prescribe or renew MHT for their patients.
Methods
We searched Medline, Embase, Scopus, and Web of Science for English language sources of the following keywords: “menopause”, “hormone therapy”, “guidelines”, and “bioidentical”. Preference was given to recently published guidelines, randomized clinical trials, and review articles. Bibliographies of these articles were also searched for relevant literature. We incorporated lessons learned from our personal experience along with the existing evidence to provide the current review.
Decision-making regarding MHT: choosing the right patient
The decision whether or not to prescribe MHT to a woman with menopause-related concerns requires a personalized discussion regarding the balance of potential risks and benefits as individualized to that woman’s health circumstances. The patient also needs to be aware of nonhormonal alternatives, including lifestyle modifications, herbal supplements, mind-body techniques, and nonhormonal prescriptions. Important factors to take into consideration include the woman’s age, type and timing of menopause, impact of symptoms on quality of life, health history, family medical history, and personal preferences. For women experiencing menopausal symptoms in their late 40s and early 50s, the overall benefit of hormones generally exceeds the risk.
Vasomotor menopausal symptoms affect approximately 75% of perimenopausal or early postmenopausal women.7 Treating moderately severe to severe menopausal symptoms is the primary indication approved by the US Food and Drug Administration (FDA) for hormone therapy in the US.4 Historically, osteoporosis was one of the primary indications for MHT use, but due to the unfavorable risk to benefit balance reported by the WHI and other clinical trials, it has been downgraded to second-line therapy.4 Treatment of vulvovaginal atrophy symptoms, reported by up to 50% of menopausal women, is also an FDA-approved indication for MHT, although topical, localized estrogen therapy is preferred for this indication. Nonvasomotor menopausal symptoms, including sleep disturbance,8 mood instability,9 difficulty with concentration, and sexual function changes10,11 also affect a substantial proportion of women during the menopausal transition,12 but have not been as extensively studied in clinical trials and are not considered primary indications for starting MHT. When women report lack of benefit for these symptoms with nonhormonal approaches, and have a poor quality of life related to nonvasomotor symptoms, MHT may be offered.
Prematurely menopausal women (<40 years) constitute a unique group in whom the general guidelines for use of MHT do not apply. In the absence of contraindications, MHT use until approximately the average age of natural menopause appears to be important for reducing the deleterious health consequences of early estrogen deprivation, including an increased risk of coronary heart disease, osteoporosis, cognitive decline, and premature death.13
The balance of benefits and risks is less favorable for women with a history of pre-existing coronary artery disease, stroke, deep venous thromboembolism, or breast cancer. For these women, MHT is ideally avoided.
Initiating MHT: choosing the optimal time
The balance of benefits and risks for MHT is most favorable within the first ten years of menopause, or up to around 60 years of age.4 During this window of opportunity, estrogen-containing hormone therapy not only relieves menopausal symptoms for women at low risk, but also may have a positive impact on women’s cardiovascular and bone health.4 MHT has been shown to slow the progression of atherosclerosis during early menopause (Figure 1), consistent with the findings of observational studies showing a reduction in the risk of coronary heart disease and total mortality.14 However, MHT initiated later in menopause may cause plaque destabilization in vessels with advanced atherosclerosis, consistent with clinical trials finding unfavorable outcomes for myocardial infarction and stroke when administered to older postmenopausal women.15 The concept of a window of opportunity is also relevant to MHT and cognitive aging, since observational data on MHT use and Alzheimer’s disease, along with randomized clinical trials data on estrogen therapy and cognitive performance in younger women, have found benefits.16,17 Likewise, women who experienced an early or premature menopause, most commonly due to bilateral oophorectomy, are now known to be at increased risk for cognitive decline or dementia and have been found to derive some protection from taking estrogen replacement.4,13
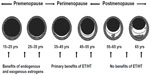  | Figure 1 Schematic representation of the natural history of coronary atherosclerosis in US women. |
Individualizing MHT: overall risks and benefits
MHT reduces the frequency and severity of hot flashes and commonly occurring symptoms such as disrupted sleep, mood instability, difficulty concentrating, and reduced quality of life.4 MHT risks and benefits beyond those of symptom relief vary depending on a woman’s age and time since menopause, such that younger women under the age of 60 years derive benefit from reduction in cardiovascular disease,18 osteoporotic fractures,19,20 type 2 diabetes,21,22 and colorectal cancer, as well as overall mortality.4 Further, the type of MHT modulates risk, because estrogen alone appears to decrease the risk of breast cancer while combination regimens with estrogen and progestogen have been shown to increase this risk after 3–5 years of use.4,23 The mode of delivery of estrogen is also important because, in contrast with oral estrogen, low-dose transdermal estrogen appears to be linked to a lower risk of cholecystitis, stroke, and deep venous thromboembolism.24
Vasomotor symptoms
Estrogen-containing MHT is the most effective treatment for hot flashes and night sweats. There is incontrovertible support for prescribing MHT to treat or manage bothersome vasomotor menopausal symptoms.25,26 Almost all systemic hormone therapy products (pills, patches, gels) are approved for the relief of vasomotor symptoms.4
Osteoporosis
MHT prevents early postmenopausal bone loss and reduces fractures in postmenopausal women.4 There is a dose-response of estrogen therapy for bone protection, but even low doses of MHT are effective in preserving or improving bone density.4 Long-term MHT use in the indication of bone preservation is considered an option for women at high risk of osteoporotic fracture, particularly when other products have been poorly tolerated, are contraindicated, or have an unfavorable risk-benefit balance.27
Cardiovascular disease and mortality
Randomized clinical trials and observational data provide evidence that estrogen-containing MHT may decrease coronary heart disease and mortality in women younger than 60 years of age and within 10 years of menopause.1,25 Thus, MHT can be safely offered to symptomatic younger menopausal women.
Some evidence suggests that estrogen therapy initiated in recently postmenopausal women slows the development of calcified atherosclerotic plaque.4 While there is accumulating evidence of mortality benefit for cardiovascular disease, MHT is not currently indicated for the prevention of coronary heart disease.4,28 In contrast, older women who are distant from the onset of menopause, have established atherosclerosis, and are given standard dose oral MHT (as in the WHI) are at increased risk for coronary heart events.1
Diabetes
In the WHI, use of MHT containing oral conjugated equine estrogens (CEE) alone or oral CEE plus progestin was found to be associated with a reduction in the development of type 2 diabetes.21,22,27 There is conflicting information about whether a protective benefit of MHT against diabetes is mediated through changes in insulin sensitivity, since some studies specifically evaluating this question have found adverse effects of MHT on insulin sensitivity,29 whereas others have found protection.30 Some evidence supports the benefit of MHT for reducing the accumulation of weight and fat mass, particularly central fat mass.27
Stroke
The effect of MHT on stroke risk is controversial. Multiple but not all controlled clinical trials and observational studies have shown that estrogen-containing MHT is associated with an increased risk of stroke, primarily ischemic stroke.4,31 There is some evidence that the risk might vary by age, but even younger women given standard-dose oral MHT have been found to be at increased risk, although the absolute risk of ischemic stroke in this population is very small.4,25
A reanalysis of the WHI data concluded that oral estrogen alone did not increase the risk of ischemic stroke in women 50–59 years of age.32 For women with early estrogen deficiency due to premature onset of menopause, there is accumulating data on protection against stroke with the administration of estrogen following oophorectomy.33 Early estrogen deprivation appears to increase a woman’s risk of stroke, and estrogen therapy may reduce this risk.33–35
Beyond the issue of age, the effect of MHT on stroke risk might vary by dose, route of administration, type of MHT, and the presence of risk factors such as hypertension. Analysis of the UK General Practice Research Database found no increased risk of stroke with low-dose transdermal estrogen use, but a slightly increased risk with oral MHT or transdermal estrogen at higher doses.36 In the Danish Nurse Cohort Study, MHT use was associated with an increased risk of stroke among hypertensive women, particularly with the use of combined estrogen plus progestogen compared with estrogen only therapy.37
Breast cancer
Evidence on the risk of breast cancer from MHT use is complex, but what is clear is that taking combination estrogen plus progestogen therapy for longer than 5 years is associated with an increased risk.6,26 The risk varies with the time of initiation relative to final menses, duration of use, body mass index, family history of breast cancer, and the type of progestogen used.27 The risk might be less with sequential compared with continuous use of progestogen, and might be less with certain progestogens, such as micronized progesterone,4 but data on this are limited to observational studies. Our current understanding is that the increased risk of breast cancer with MHT use likely results from MHT promoting the growth of pre-existing cancers that might not have grown otherwise or might have remained too small to be diagnosed.4
Use of estrogen alone was reported to be associated with no increase or even a decrease in risk of breast cancer in the WHI study evaluating estrogen alone compared with placebo over a median interval of 7 years in women who had previously undergone hysterectomy.38 The Million Women Study, by contrast, found an increase in risk of breast cancer among women who started estrogen only therapy within 5 years of onset of menopause, with a magnitude of risk of 13 additional cases per 10,000 women per year of use.39,40 The Nurses’ Health Study also found an increased risk with longer-term use of estrogen only therapy, with a relative risk of 1.3 for 5–9 years of use, 1.2 for 10–14 years of use, and 1.6 for more than 15 years of use.41–43 Concern about breast cancer risk continues to be a major factor prompting the recommendation to limit MHT use to the shortest duration needed for symptom relief.
Venous thromboembolism
A previous history of deep venous thromboembolism is generally considered a contraindication to systemic hormone therapy use. Systemic MHT increases the risk of venous thromboembolic events such as deep vein thrombosis and pulmonary emboli by around 2–4-fold, depending on the route of administration and potentially the type and dose of hormone product used.4,44 However, in women 50–59 years of age, the baseline risk of deep venous thromboembolism is low, so the absolute risk of venous thromboembolic events with hormone therapy use is rare.
The risk for deep venous thromboembolism is considered to be greater with oral than nonoral routes of administration, consistent with our knowledge that oral estrogen increases the production of thrombotic proteins by its first-pass hepatic effect. A similar increase in thrombotic protein synthesis does not occur with transdermal estrogen therapy administration. Well designed case-control studies report no increase in risk of deep venous thromboembolism with transdermal compared with oral estrogen therapy, even in women at markedly increased risk, such as those with a Factor V Leiden mutation who carry a seven-fold increased risk of deep venous thromboembolism with oral estrogen + progestin use.45–47
Cognition
The concept that MHT might provide protection against cognitive aging is controversial but intriguing. Observational studies in younger menopausal women using MHT have shown a reduced risk of cognitive decline and a reduction in risk of Alzheimer’s dementia by 29%–44%.48 Studies in prematurely menopausal women have also supported the role of MHT in preventing cognitive decline and dementia.49,50 By contrast, randomized controlled trials in older women, such as those included in the Women’s Health Initiative Memory Study, showed an increased risk of dementia in women aged 65 years and older who were given MHT.51
Drawing from this understanding of the variable effects of MHT on cognition based on age or time since menopause, initiation of MHT is generally avoided in women who are later in menopause, as the risk of cognitive impairment likely exceeds benefits. In contrast, for women who experience premature menopause, MHT use until the average age of natural menopause may offer some protection against cognitive decline. In women undergoing natural menopause at an average age, the role of MHT in providing cognitive protection versus causing harm remains unclear.4,16,48
Mood
The relationship between mood and menopause is not completely understood. Clinical trials have not revealed a direct association between menopause and mood instability, although many women experience mood swings that are thought to be secondary to fluctuations in the levels of ovarian hormones. A multitude of other variables, including stress and sleep disturbance, may also predispose a woman to mood disruption during menopausal transition.52 Women with a prior history of premenstrual syndrome may experience worsening mood swings during perimenopause, resulting in more severe premenstrual syndrome. Women with a previous history of depression may be particularly vulnerable to recurrent depression requiring antidepressant treatment and/or counseling during this phase of life.4
Urogenital atrophy
Up to 50% of postmenopausal women experience symptoms related to urogenital atrophy,53 while only 25% women seek treatment for their symptoms.54–56 Many women are reluctant to bring up the topic with their health care providers due to embarrassment53 or the belief that topical estrogen therapy carries the same risks as systemic MHT.55
Urogenital atrophy may result in vaginal dryness, itching, burning, dyspareunia, urinary frequency, urgency, urge incontinence, and recurrent urinary tract infections, thereby affecting women’s quality of life negatively and causing low self-esteem.55 Topical estrogen therapy improves vaginal thickness, elasticity, lubrication, and blood flow, favorably affects vaginal pH and microflora, and improves sexual response. It alleviates vaginal dryness, soreness, irritation, pruritus, and dyspareunia.57 Local estrogen therapy also alleviates urinary symptoms associated with atrophy, particularly urge incontinence and recurrent urinary tract infections.58
With local vaginal estrogen therapy, some absorption of estrogen into the systemic circulation occurs, particularly early in treatment when the vaginal mucosa is thinned and atrophic. Once vaginal maturation and thickening occur, absorption is reduced.56 Low-dose vaginal estrogen stimulates the endometrium minimally, such that progestogens are not required for protection against endometrial proliferation. Vaginal estrogens are available in several formulations that are comparable in efficacy. Hence, the choice of regimen is generally based on patient preference (Table 1).
  | Table 1 Nonoral estrogen and progestogen products available in the US |
Prescribing MHT: need for diagnostic evaluation
Menopause is a clinical diagnosis and no laboratory testing is required before initiating MHT. Checking levels of estradiol, progesterone, and follicle-stimulating hormone is not necessary and generally provides no meaningful information.
Prescribing MHT: choice of route and regimen
Estrogen therapy
Estrogen only MHT (estrogen therapy) is utilized for women who have undergone hysterectomy, whereas MHT with estrogen plus progestogen is indicated for women with an intact uterus. In women who have undergone endometrial ablation, the risk of endometrial cancer persists, hence estrogen plus progestogen is recommended.59,60
Initiation of systemic estrogen therapy requires a review of the types and regimens of available preparations. Underlying health concerns and personal preferences guide choice of the preferred regimen. CEEs, synthetic esterified estrogens, ethinyl estradiol-containing preparations, or pure 17-beta estradiol-containing products are the estrogens commonly used in MHT preparations in standard or low-dose formulations (Tables 1 and 2).61
  | Table 2 Oral estrogen and progestogen products available in the US |
Bioidentical estrogens are plant-derived exogenous estrogens that are biochemically the same as endogenous ovarian estrogens. Bioidentical estrogens can be available either as an FDA-approved prescription product, or they can be obtained from compounding pharmacies that are not federally regulated for purity, potency, efficacy, or safety of hormones. The benefits and risks of estrogens in general apply to all synthetic as well as compounded and FDA-approved bioidentical preparations.62,63
Estrogens can be administered via the oral route (eg, CEE, esterified estrogens, ethinyl estradiol, 17 beta-estradiol) or via the nonoral route (eg, 17-beta estradiol). Both oral and transdermal estrogens provide symptom benefit for menopausal symptoms and have bone-sparing effects with equal efficacy.64 Other health effects of estrogens, however, are quite variable based on the route of administration. Unlike their nonoral counterparts, oral estrogens undergo hepatic first-pass metabolism, which results in lower bioavailability and a need for higher dosing.64 Oral estrogens increase the hepatic production of sex hormone-binding globulin with associated lowering of free testosterone, potentially adversely affecting sex drive and sexual responsiveness.65 Oral estrogens also stimulate other hepatic enzymes, which can affect the cardiovascular, thrombotic, and vascular systems. These important clinical considerations guide the choice of MHT (Table 3).64,66
  | Table 3 Comparative effects of oral versus transdermal estrogens |
Progestogen therapy
The primary indication for progestogen use in MHT is to provide protection against endometrial hyperplasia or endometrial cancer.4 Progestogens are a broad group of progestational compounds that includes micronized progesterone, which is bioidentical to endogenous progesterone, and synthetic progestins such as medroxyprogesterone acetate (MPA, a C-21 derivative), and norethindrone (a C-19 derivative, Tables 1 and 2).
Progestogens currently available for MHT are similar in their ability to protect against excess estrogen stimulation of the endometrium, but differ in some of their nonendometrial effects, such as those on metabolism, the vascular system, breast, and mood.67 For example, micronized progesterone as opposed to MPA does not negate the favorable effects of oral estrogen on lipids.68 Micronized progesterone as opposed to MPA also does not increase glucose levels when combined with oral CEE.68 Smaller controlled studies have shown a positive effect of micronized progesterone on sleep,69 mood,70 and fluid balance diuresis,71 in contrast with MPA which may cause depression and fluid retention.
The potential role of progestogens in increasing the MHT-associated breast cancer risk has come under greater scrutiny, particularly since the WHI trial showed an increased risk of breast cancer after continuous use of CEE and MPA for 5 years,6,26 compared with CEE alone which showed no increased risk.38 The recently completed multicenter, randomized Kronos Early Estrogen Prevention Study showed no increase in breast cancer in the hormone groups (CEE + micronized progesterone or estradiol + micronized progesterone) after 4 years of MHT.72 The French Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale (E3N) cohort study found that the risk of breast cancer was lower with micronized progesterone and dydrogesterone regimens (relative risk of 1) compared with other progestins (combined relative risk for MPA and other progestins of 1.69).73 Thus, some have proposed that micronized progesterone may be a safer alternative to MPA with regard to breast cancer risk.
Micronized progesterone and MPA can be prescribed in cyclic or continuous oral dosing regimens. A vaginal gel containing micronized progesterone is also available, Crinone® 4%; 45 mg (Watson Pharmaceuticals Inc., Dublin, Ireland), although it is not FDA-approved for postmenopausal use.74 Transdermal use of micronized progesterone is not recommended for endometrial protection concurrent with systemic estrogen therapy because the absorption is variable and unpredictable.75 The levonorgestrel-containing intrauterine device (Mirena®, Bayer HealthCare Pharmaceuticals Inc., Montville, NJ, USA), although not approved by the FDA for menopausal use, is also sometimes used as an alternative for endometrial protection with estrogen-containing MHT in women who are still menstruating or those who do not tolerate oral micronized progesterone or MPA well.76
Ongoing MHT: monitoring use
Menopause practitioners often report that bringing patients back within a few months after initiating MHT may be useful to assess symptom response and tolerance, allowing for dose adjustment if needed. Thereafter, annual reassessment of the balance of benefits and risks individualized to each woman’s particular health circumstances is generally advised. Annual reassessment typically includes a clinical breast examination, mammogram (for women 40 years and older), pelvic examination (if indicated), symptom assessment, review of intervening health concerns, family medical history, discussion of any new research findings, and reassessment of the woman’s preferences.
Discontinuing MHT: choosing the optimal time
The current practice is to limit MHT use to the shortest interval and lowest dose needed for symptom relief or to achieve treatment goals.4 For women who experience menopause around the average age, an increased risk of breast cancer with longer-term combined MHT use guides the general recommendation to limit therapy to 3–5 years. Women who have undergone hysterectomy and take estrogen alone are not constrained by the same 5-year recommendation based on WHI results showing no increase in breast cancer risk with estrogen only therapy for a median interval of 7 years.4 For women with a history of premature or early-onset menopause, continuing MHT until at least the average age of natural menopause is generally advised, and is based on the need for symptom relief thereafter.4,6
Extending MHT use for longer intervals is considered acceptable for some women, provided that the woman is fully informed as to the potential risks and has appropriate clinical supervision.4 This may include women at high risk of osteoporotic fracture, for whom alternate therapies are not appropriate or tolerated. This may also include women who have failed previous attempts to stop MHT and who, after discussing the pros and cons of MHT with their provider, have determined that the benefits of menopause symptom relief outweigh the risks for their particular situation.4
There is no single best way to discontinue MHT. Vasomotor symptoms tend to recur in 50% women, independent of their age or duration of MHT use.4 Studies have found no advantage to tapering versus abrupt discontinuation of MHT,77 so individual preferences commonly guide the decision on how to stop hormone therapy.
Conclusion
Age and time since menopause affect the balance of benefits and risks for hormone therapy use in postmenopausal women. For women who experience premature or early-onset menopause, estrogen therapy should generally be administered until around the average age of natural menopause. For healthy women experiencing menopausal symptoms around the average age of natural menopause, MHT provides excellent symptom relief and poses low risk. Withholding MHT from symptomatic women might pose a risk, particularly with regard to cardiovascular disease and osteoporosis (Figure 2). On the contrary, MHT may be associated with increased risk when initiated in older women and is generally avoided. The wealth of clinical trial data in recent years, while sometimes daunting to the prescribing provider, not only allows for but begs for personalization of decision-making about hormone therapy in order to optimize care for women with menopausal concerns.
  | Figure 2 (A) Risks and benefits of MHT (expressed as attributable or excess risk) in women starting MHT between the ages of 50 and 59 years or less than 10 years after the start of menopause. Figure expanded from panel B for clear visualization. (B) Number of women expected to get hot flashes and vaginal dryness symptom benefit per 1,000 women taking MHT for 5 years. Design of panels A and B is the same. Panel B compares the number of women benefiting from relief of symptoms of hot flashes and vaginal atrophy with the number of women experiencing other risks and benefits. |
It is important to keep the perspective that MHT is a tool that affects the care of menopausal women not only during their transition years, but also over the long-term given that they spend one third of their lives in menopause. Since a large proportion of menopausal women will suffer the consequences of cardiovascular disease and osteoporosis, further research regarding the role of MHT in these chronic medical conditions is needed. The science of MHT is evolving, and it is important to stay informed and keep an open perspective as our understanding about these agents improves.
Disclosure
The authors report no conflicts of interest in this work.
References
Lobo RA. Where are we 10 years after the Women’s Health Initiative? J Clin Endocrinol Metab. 2013;98(5):1771–1780. | |
Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. | |
Hodis HN, Collins P, Mack WJ, Schierbeck LL. The timing hypothesis for coronary heart disease prevention with hormone therapy: past, present and future in perspective. Climacteric. 2012;15(3):217–228. | |
North American Menopause Society. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause. 2012;19(3):257–271. | |
Panay N, Hamoda H, Arya R, Savvas M; British Menopause Society, Women’s Health Concern. The 2013 British Menopause Society and Women’s Health Concern recommendations on hormone replacement therapy. Menopause Int. 2013;19(2):59–68. | |
de Villiers TJ, Pines A, Panay N, et al. Updated 2103 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric. 2013;16:316–337. | |
Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96(7):1226–1235. | |
Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33(4):539–549. | |
Freeman EW. Associations of depression with the transition to menopause. Menopause. 2010;17(4):823–827. | |
Lamont J. Female sexual health consensus clinical guidelines. J Obstet Gynaecol Can. 2012;34(8):769–775. | |
Gregersen N, Jensen PT, Giraldi AE. Sexual dysfunction in the peri- and postmenopause. Status of incidence, pharmacological treatment and possible risks. A secondary publication. Dan Med Bull. 2006;53(3):349–353. | |
[No authors listed]. NIH State-of-the-Science Conference Statement on management of menopause-related symptoms. NIH Consens State Sci Statements. 2005;22(1):1–38. | |
Shuster LT, Rhodes DR, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2009; 65(2):161–166. | |
Hodis HN, Mack WJ. Hormone replacement therapy and the association with coronary heart disease and overall mortality: clinical application of the timing hypothesis. J Steroid Biochem Mol Biol. July 9, 2013. [Epub ahead of print.] | |
Clarkson TB, Melendez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20(3):342–353. | |
Maki P. Is timing everything? New insights into why the effect of estrogen therapy on memory might be age dependent. Endocrinology. 2013;154(8):2570–2572. | |
Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm Behav. 2013;63(2):231–237. | |
Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. | |
Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290(13):1729–1738. | |
Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. | |
Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. | |
Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9. | |
Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289(24):3243–3253. | |
Simon JA. What’s new in hormone replacement therapy: focus on transdermal estradiol and micronized progesterone. Climacteric. 2012; 15 Suppl 1:3–10. | |
de Villiers TJ, Gass ML, Haines CJ, et al. Global Consensus Statement on menopausal hormone therapy. Maturitas. 2013;74(4):391–392. | |
Stuenkel CA, Gass ML, Manson JE, et al. A decade after the Women’s Health Initiative – the experts do agree. Fertil Steril. 2012;98(2):313–314. | |
Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(7 Suppl 1):s1–s66. | |
Valdiviezo C, Lawson S, Ouyang P. An update on menopausal hormone replacement therapy in women and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2013;20(2):148–155. | |
Sites CK, L’Hommedieu GD, Toth MJ, Brochu M, Cooper BC, Fairhurst PA. The effect of hormone replacement therapy on body composition, body fat distribution, and insulin sensitivity in menopausal women: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90(5):2701–2707. | |
Zhu L, Brown WC, Cai Q, et al. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes. 2013;62(2):424–434. | |
Henderson VW, Lobo RA. Hormone therapy and the risk of stroke: perspectives 10 years after the Women’s Health Initiative trials. Climacteric. 2012;15(3):229–234. | |
LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314. | |
Rocca WA, Grossardt BR, Miller VM, Shuster LT, Brown RD Jr. Premature menopause or early menopause and risk of ischemic stroke. Menopause. 2012;19(3):272–277. | |
Rivera CM, Grossardt BR, Rhodes DJ, Rocca WA. Increased mortality for neurological and mental diseases following early bilateral oophorectomy. Neuroepidemiology. 2009;33(1):32–40. | |
Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113(5):1027–1037. | |
Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. | |
Løkkegaard E, Jovanovic Z, Heitmann BL, et al. Increased risk of stroke in hypertensive women using hormone therapy: analyses based on the Danish Nurse Study. Arch Neurol. 2003;60(10):1379–1384. | |
Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295(14):1647–1657. | |
Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. | |
Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305. | |
Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332(24):1589–1593. | |
Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol. 2000;152(10):950–964. | |
Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166(9):1027–1032. | |
Canonico M, Oger E, Plu-Bureau G, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115(7):840–845. | |
Scarabin PY, Oger E, Plu-Bureau G; EStrogen and THromboEmbolism Risk Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362(9382):428–432. | |
Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227–1231. | |
Laliberte F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052–1059. | |
Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20(6):695–709. | |
Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. | |
Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13(4):345–357. | |
Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. | |
Soares CN. Can depression be a menopause-associated risk? BMC Med. 2010;8:79. | |
MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87–94. | |
North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14(3 Pt 1):355–369. | |
Simon JA, Kokot-Kierepa M, Goldstein J, Nappi RE. Vaginal health in the United States: results from the Vaginal Health: Insights, Views and Attitudes survey. Menopause. 2013;20(10):1043–1048. | |
Sturdee DW, Panay N, International Menopause Society Writing Group. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric. 2010;13(6):509–522. | |
Simon JA, Maamari RV. Ultra-low-dose vaginal estrogen tablets for the treatment of postmenopausal vaginal atrophy. Climacteric. 2013;16 Suppl 1:37–43. | |
Cody JD, Richardson K, Moehrer B, Hextall A, Glazener CM. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev. 2009;4:CD001405. | |
Shapiro S, Kelly JP, Rosenberg L, et al. Risk of localized and widespread endometrial cancer in relation to recent and discontinued use of conjugated estrogens. N Engl J Med. 1985;313(16):969–972. | |
AlHilli MM, Hopkins MR, Famuyide AO. Endometrial cancer after endometrial ablation: systematic review of medical literature. J Minim Invasive Gynecol. 2011;18(3):393–400. | |
Crandall C. Low-dose estrogen therapy for menopausal women: a review of efficacy and safety. J Womens Health (Larchmt). 2003;12(8):723–747. | |
Boothby LA, Doering PL, Kipersztok S. Bioidentical hormone therapy: a review. Menopause. 2004;11(3):356–367. | |
Sood R, Shuster L, Smith R, Vincent A, Jatoi A. Counseling postmenopausal women about bioidentical hormones: ten discussion points for practicing physicians. J Am Board Fam Med. 2011;24(2):202–210. | |
Goodman MP. Are all estrogens created equal? A review of oral vs transdermal therapy. J Womens Health (Larchmt). 2012;21(2):161–169. | |
Arlt W. Androgen therapy in women. Eur J Endocrinol. 2006;154(1):1–11. | |
Minkin MJ. Considerations in the choice of oral vs transdermal hormone therapy: a review. J Reprod Med. 2004;49(4):311–320. | |
Fitzpatrick LA, Good A. Micronized progesterone: clinical indications and comparison with current treatments. Fertil Steril. 1999;72(3):389–397. | |
Barrett-Connor E, Slone S, Greendale G, et al. The Postmenopausal Estrogen/Progestin Interventions Study: primary outcomes in adherent women. Maturitas. 1997;27(3):261–274. | |
Montplaisir J, Lorrain J, Denesle R, Petit D. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause. 2001;8(1):10–16. | |
de Lignieres B. Oral micronized progesterone. Clin Ther. 1999;21(1):41–60. | |
Sitruk-Ware R, Bricaire C, De Lignieres B, Yaneva H, Mauvais-Jarvis P. Oral micronized progesterone. Bioavailability pharmacokinetics, pharmacological and therapeutic implications – a review. Contraception. 1987;36(4):373–402. | |
The North American Menopause Society. KEEPS Results Give New Insight Into Hormone Therapy. 2012. Available from: http://www.menopause.org/annual-meetings/2012-meeting/keeps-report. Accessed October 5, 2013. | |
Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107(1):103–111. | |
de Ziegler D, Ferriani R, Moraes LA, Bulletti C. Vaginal progesterone in menopause: Crinone 4% in cyclical and constant combined regimens. Hum Reprod. 2000;15 Suppl 1:149–158. | |
Lewis JG, McGill H, Patton VM, Elder PA. Caution on the use of saliva measurements to monitor absorption of progesterone from transdermal creams in postmenopausal women. Maturitas. 2002;41(1):1–6. | |
Sitruk-Ware R. The levonorgestrel intrauterine system for use in peri- and postmenopausal women. Contraception. 2007;75(Suppl 6):S155–S160. | |
Haimov-Kochman R, Barak-Glantz E, Arbel R, et al. Gradual discontinuation of hormone therapy does not prevent the reappearance of climacteric symptoms: a randomized prospective study. Menopause. 2006;13(3):370–376. | |
Mikkola TS, Clarkson TB, Notelovitz M. Postmenopausal hormone therapy before and after the Women’s Health Initiative study: what consequences? Ann Med. 2004;36(6):402–413. | |
Santen RJ, Allred Dc, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J. Clin Endocrinol Metab. 2010 Jul;95(7 Suppl 1):s1–s66. doi:10.1210/jc.2009–2509. Epub Jun 21, 2010. | |
The North American Menopause Society (menopause.org) [homepage on the Internet]. Hormone Products for Postmenopausal Use in the United States and Canada. Available from http://www.menopause.org/docs/professional/htcharts.pdf?sfvrsn=6. Accessed December 11, 2013. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
