Back to Journals » Clinical Ophthalmology » Volume 9
Potential role of lampalizumab for treatment of geographic atrophy
Authors Rhoades W, Dickson D, Do D
Received 12 September 2014
Accepted for publication 18 November 2014
Published 11 June 2015 Volume 2015:9 Pages 1049—1056
DOI https://doi.org/10.2147/OPTH.S59725
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
This paper has been retracted.
William Rhoades, Drew Dickson, Diana V Do
Truhlsen Eye Institute, Department of Ophthalmology and Visual Sciences, University of Nebraska Medical Center, Omaha, NE, USA
Abstract: The purpose of this article is to review the pathways underlying age-related macular degeneration and potential therapeutic targets, focusing on the complement pathway and the recent MAHALO Phase II trial of the investigational drug lampalizumab. This trial was the first to have shown positive results for the treatment of geographic atrophy in age-related macular degeneration. It has potential as a future treatment, and is currently undergoing a Phase III trial.
Keywords: age-related macular degeneration, complement inhibition, AMD
Introduction/background
Age-related macular degeneration (AMD) is the leading cause of blindness in the United States, and it has been projected that the prevalence will double in the next 10 years.1 The advanced forms of AMD, which may cause loss of central vision, include neovascular or “wet” AMD and non-neovascular or “dry” AMD. These advanced stages of AMD affect approximately 1.6% of Americans over the age of 55 years, and over 13% of Americans over the age of 85 years.1,2 There are approximately 8 million people in the United States with intermediate forms of AMD that put them at risk for disease progression.3 There is a great need for effective treatments for AMD, and therefore there are many active studies seeking to further medical knowledge into the pathophysiology and genetics of this disease process.
Treatments exist for neovascular AMD; however, these patients make up only a small fraction (10%–15%) of AMD patients.1 There remains no proven option for treating geographic atrophy (GA) in non-neovascular AMD.4–7 The Age-Related Eye Disease Study (AREDS) and AREDSII have confirmed the benefit of prophylactic vitamins in certain individuals.8,9 However, the results have been less significant compared to the advances in neovascular AMD. If GA progresses to the foveal center, these patients experience devastating visual loss. GA not involving the foveal center can cause other visual disturbances such as paracentral scotomas, which can impair vision in dim light, diminish contrast sensitivity, and limit reading ability.10–12 Studies have shown that patients with paracentral GA typically experience progression, with risk of loss of visual acuity.11
Genetics and AMD
Much of the advances in understanding of non-neovascular AMD have been in the realms of genetics and pathophysiology. While nongenetic factors such as age, diet, and smoking are known to play a role in the development of AMD, recently discovered genetic factors provide key insight into potential non-neovascular AMD treatments. It is theorized that 20 known AMD associated genes can explain 40%–60% of disease heritability. The genes with the largest share of genetic risk for AMD include CFH, C2/CFB, and ARMS2/HTRA1. Identified in 2005, the CFH gene codes for complement factor H (CFH), a key component in the alternative pathway which protects self-cells from destructive inflammation through the complement cascade.13 CFH regulates this inflammatory pathway, by inhibiting proinflammatory protein C3b. Studies have suggested that homozygosity for CFH increases the likelihood of AMD by a factor of 7.4.14
Single nucleotide polymorphisms (SNPs) in the CFH and CFB genes contribute to the progression of GA. Although there are multiple SNPs of the CFH gene that are associated with developing AMD, the Y402H SNP is the most common variant.15 This SNP alters Factor H binding to Bruch’s membrane, which is hypothesized to lead to poorly controlled complement turnover and a state of localized, excessive, chronic inflammation.16 Approximately 30% of those of European descent carry at least one copy of the Y402H SNP. Heterozygotes are 2.3 times more likely to develop AMD, while homozygotes are 5.2 times more likely to develop AMD than those without the Y402H allele.17 The C2/CFB polymorphisms typically occur as one of two different haplotypes, both which have shown the ability to decrease the risk of developing AMD in Caucasians.15 The L9H variant in complement factor B (CFB) is strongly linked with either the E318D variant in C2 or the R32Q variant in C2. These polymorphisms are thought to decrease complement activation and therefore decrease the risk of AMD progression.15 The ARMS2/HTRA1 polymorphism is also associated with an increased risk of developing AMD.18 These polymorphisms are strongly linked.19 The contribution of this mutation to the pathogenesis of AMD is not completely understood, but an ARMS2 mutation is thought to only participate in non-neovascular AMD.19 The most common ARMS2 variant is a SNP called Ala69Ser.18 Its presence increases the risk of developing AMD by a factor of seven. Together, the Y402H SNP of the CFH gene and the Ala69Ser SNP may explain up to 75% of the genetic risk of AMD.20 Many other genetic variations exist that are associated with an increased risk of developing AMD, with a large number of these polymorphisms found in the complement cascade. Besides the common CFH and CFB/C2 polymorphisms, gene alterations coding for the complement system that are associated with AMD include mutations to CFH-related proteins, C3, complement factor I, and C9. Each of these genes possesses multiple different possible polymorphisms.
The complement system and AMD
The complement system is part of the body’s innate immune system, the portion of the body’s defense from foreign pathogens that is nonspecific to the bodily insult. It includes mast cells, eosinophils, basophils, microglial cells, and phagocytes such as macrophages and polymorphonuclear leucocytes. This is in contrast to the adaptive immune system, which uses T and B lymphocytes to recognize foreign antigens and create long lasting immunity through antibody production.
The complement system is considered part of the humoral component of the immunity-related nonspecific inflammatory cascade. It works by inducing inflammation, opsonizing foreign pathogens, destroying foreign cells, and removing neutralized foreign pathogens. There are three main complement pathways. In the classic complement pathway, antibody-coated targets and antigen-antibody complexes cause the Fc receptor of antigen-activated antibody molecules to bind and activate C1. In the alternative pathway, microbial surface constituents such as polysaccharides activate C3 convertase, which causes proteolysis of C3. In the mannose-binding lectin pathway, lectin can bind to mannose residues on pathogens such as viruses and also activate C3 convertase. Phagocytes have complement receptors that can bind C3b on the microbial surface, and which promotes phagocytosis. Other complement cascade molecules include C5a and C3a, which are involved in phagocytic chemotaxis, and stimulating mast cell-mediated inflammation via histamine release. The classic and alternative pathways are involved in forming the membrane attack complexes involving C5 to C9, which can destroy microbes through osmotic lysis.21
The first indication that complement was involved in AMD initiation and/or progression was the discovery of complement byproducts in drusen.15,22 This led to the discovery of associations between complement dysregulation and AMD.23 Given the significant prevalence of complement mutations in the AMD population, the complement cascade makes an intriguing clinical therapeutic target for non-neovascular AMD. Studies have already demonstrated that SNPs to the CFH and CFB genes contribute to the progression of GA.24 However, despite the promise that complement blockade shows for potential therapies, there have been several unsuccessful Phase I and Phase II trials of monoclonal antibodies, aptamers, receptor agonists, and a compstatin derivative which purported to modify the complement cascade. All have failed to show proof of concept that blocking or altering the complement cascade can provide therapeutic results.25 Studies have tried to reduce complement deposits, block C3, and block C5, all without positive results. Table 1 shows a list of recently investigated complement modifying therapies.
  | Table 1 Treatments for age-related macular degeneration that modulate the complement system |
Current breadth of AMD treatments under investigation
As progress has been made in understanding the pathophysiology of AMD, new treatment strategies are being examined. Current research is aimed at visual cycle inhibitors, such as fenretinide (SirionTherapeutics, Tampa, FL, USA), ACU-4429 (Acucela, Inc, Bothell, WA, USA), and ALK-001 (Alkeus Pharmaceuticals, Boston, MA, USA), which work by down-regulating the visual cycle to decrease the accumulation of the toxic waste products of retinal metabolism. Other research involves RN6G (PF-4382923, Pfizer, Inc, New York, NY, USA) and GSK933776 (GlaxoSmithKline, Research Triangle Park, NC, USA), which regulate the accumulation of amyloid β, which has been found in drusen.26 Other research is ongoing into neuroprotective drugs, such as the prostaglandin analog UF-021 (isopropyl unoprostone; Ocuseva; Sucampo Pharmaceuticals, Japan), ciliary neurotrophic factor (CNTF/NT501; Neurotech, Lincoln RI, USA), a serotonin 1A agonist, tandospirone (AL-8309B; Alcon Research Ltd, Fort Worth, TX, USA), and brimonidine tartrate intravitreal implant (Allergan, Irvine, CA, USA). MC-1101 (MacuCLEAR, Inc, Plano, TX, USA) is an experimental topical agent which proposes to slow AMD by increasing choroidal perfusion. Stem cell research may also play a role in treating GA and AMD. Agents such as HuCNS-SC (StemCells Inc, Newark, CA, USA) and MA09-hRPE (Advanced Cell Technology, Santa Monica, CA, USA) are being tested as possible stem cell treatments for GA.26
Anti-inflammatory treatments under study
The only treatment for GA that has shown positive results in clinical trials at this point in time has been the anti-inflammatory treatment lampalizumab (Genentech/Roche, South San Francisco, CA, USA). However, other anti-inflammatory treatments have been trialed or are currently under investigation. Fluocinolone (Iluvien; Alimera Sciences, Alpharetta, GA, USA) is a steroid. Glatiramer acetate (Copaxone; Teva Pharmaceuticals, Kfar-Saba, Israel) is an anti-inflammatory drug aimed at decreasing amyloid-related inflammation. Sirolimus (Rapamycin; Wyeth, Madison, WI, USA) is an antifungal which has yet to show positive results, though testing is ongoing. Another complement inhibitor which inhibits the action of C5, LFG316 (Novartis Pharmaceutical Corporation, East Hanover, NJ, USA) is currently under study. POT-4 (Appellis Pharmaceuticals, Crestwood, KY, USA and Alcon Research Ltd) acts by inhibiting the formation of C3a and C3b from C3, inhibiting the classic, alternative, and mannose-binding lectin pathways of the complement system. Eculizumab (SOLIRIS, Alexion Pharmaceuticals, Cheshire, CT, USA) is an inhibitor of C5 that prevents the formation of the membrane attack complexes at the bottom of the complement cascade. Recent investigation has not met its primary endpoint.27 ARC-1905 (Ophthotech, Princeton, NJ, USA) also targets C5, and has been studied in AMD patients.26
Factor D
Factor D is a critical early component of the alternative pathway that involves CFH. Factor D serves as the rate-limiting step of the alternative pathway, and it is present in lower plasma concentrations than other complement factors.28 Factor D is responsible for cleaving its substrate, Factor B, prior to its association with C3. After factor D-mediated cleavage, factor B converts into the proteolytically active factor Bb that initiates the alternative pathway and activates important convertases.28,29 Factor D is upstream of factor B and other critical AMD-associated proteins, including C3, CFH, and CFI.30 Recent analyses of single nucleotide polymorphisms within the factor D have shown increased factor D levels in AMD patients compared with controls.31
Lampalizumab
Lampalizumab (Genentech/Roche) is an antigen-binding fragment derived from a humanized monoclonal antibody to factor D. A Phase III trial of lampalizumab, a factor D inhibitor, is currently underway. Factor D was selected as a target given its location in the complement cascade, and it is presence in lower abundance than C3. The Phase I clinical trial was an open-label safety trial individuals 18 patients who underwent intravitreal injection of the study drug. No safety signals were identified in this small prospective study. Inclusion criteria for the Phase I trial allowed only patients aged 50 years to 85 years with GA secondary to AMD in the absence of choroidal neovascularization for the study eye. Patients were given an injection of lampalizumab in the study eye at Day 0. Doses used for each cohort (three patients per cohort) were 0.1, 0.5, 1, 2, 5, and 10 mg in a volume of 0.1 mL. The cohort with the lowest dose was filled with patients first, and patients were monitored over a period of 90 days to ensure safety and tolerability. After 14 days without any adverse events, the cohort with the next highest dose was filled, and the cycle of injection and monitoring was repeated this way for each of the subsequent cohorts. There were no ocular or systemic adverse events or serious adverse events related to lampalizumab observed in these patients. The most common ocular adverse events unrelated to lampalizumab were subconjunctival hemorrhage and foreign body sensation, which were attributed to the intravitreal injection. Additional adverse events unrelated to the Phase I lampalizumab trial are included in Table 2. Since no dose-limiting toxicities were reported for the six cohorts and 10 mg was the highest dose tested, 10 mg was determined to be the maximum tolerated dose of lampalizumab. Because of this, the 10 mg dose was chosen to be used in the Phase II testing of lampalizumab for GA.28 The Phase II trial testing Factor D inhibition in AMD is called MAHALO.
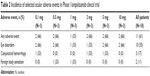  | Table 2 Incidence of selected ocular adverse events in Phase I lampalizumab clinical trial |
The MAHALO Phase II trial
Study design and methods
MAHALO tested lampalizumab dosed monthly or bimonthly against sham injections. Patients were divided into two sham injection groups (monthly and bimonthly, both with N=21), and two treatment groups receiving lampalizumab 10 mg monthly (N=43) and bimonthly (N=44). Treatments in all four subgroups were concluded after 18 months in all 129 patients. After 18 months, a 3-month observation was made for safety purposes, and patients could continue treatments after 18 months in an open-label extension study. The study schema is shown in Figure 1.
  | Figure 1 MAHALO Phase II study design. |
The primary endpoint for the Phase II trial was mean change in geographic area from baseline to month 18 on fundus autofluorescence. The study was only powered to look at its primary endpoint. However, secondary endpoints were mean change in geographic area assessed by color fundus photographs, and mean change from baseline in Early Treatment Diabetic Retiropathy Study (ETDRS) best corrected visual acuity (BCVA). The authors also looked at mean change from baseline in GA area within three subgroups of patients: those who had less than 10 mm2 of GA, those who started with more than 10 mm2 of GA, and those with genetic markers determined prior to randomization. Baseline size of GA was taken into account when randomizing patients, and comparisons between the two treatment arms were made against the two sham groups pooled together.
A large percentage of patients discontinued the study prior to month 18. Seven sham patients, eleven monthly patients, and 12 bimonthly patients did not complete the study. A modified intention to treat strategy was used to analyze the data. It appears that two of the seven patients who did not complete the study from the sham group were excluded. Two of the eleven patients who did not finish the study from the monthly group were excluded. Three of the twelve patients who did not finish the study were excluded from the bimonthly treatment group.
Inclusion and exclusion criteria
Inclusion criteria for MAHALO included bilateral GA secondary to age-related macular degeneration in the absence of neovascularization. BCVA by the ETDRS testing was between 20/50 and 20/400 (Snellen equivalent). GA had to be between one and seven disc areas (2.5 mm2 to 17.5 mm2). If GA was multifocal, then at least one lesion had to be greater than half a disc area. Patients also had to show hyperautofluorescence adjacent to their GA. Exclusion criteria included previous treatment with any intravitreal agent, history of any retinal surgery, or any retinal therapeutic procedures. Patients were also excluded if they had GA due to any cause other than age-related macular degeneration.
Patient demographics
Patient demographics are shown in Table 3. The treatment and sham groups were demographically similar. Mean age for the pooled sham group was 78.5 years with a standard deviation of 7.3 years. The monthly lampalizumab group was 80.4 years with a standard deviation of 7.2 years. The bimonthly lampalizumab group was aged 77.4 years with a standard deviation of 7.3 years. Sixty percent of patients in the sham group were female. Sixty-six percent of patients in the monthly treatment group were female, and 43.9% of patients in the bimonthly treatment group were female. Almost all of the patients were Caucasian.
  | Table 3 MAHALO patient demographics |
Baseline characteristics of enrolled patients
Baseline outcome measures are shown in Table 4. Average ETDRS letters were similar at baseline in the three comparison groups, being 45.9 (13.4), 47.6 (12.8), and 49.5 (11.0) in the sham, monthly, and bimonthly groups, respectively (with standard deviation). Median Snellen equivalent was also similar, with 20/125, 20/100, and 20/100 in the sham, monthly, and bimonthly groups, respectively. The average total area of GA was 8.85 mm2, 8.56 mm2, and 8.56 mm2 in the sham, monthly, and bimonthly treatment groups, with standard deviations of 4.18 mm2, 3.86 mm2, and 4.90 mm2, respectively.
Results
Analysis of the primary outcome measure, change in GA size, revealed that at the study endpoint, there was a 20.4% reduction in mean change from baseline in GA area with a P-value less than the prespecified significance level of 0.2. A positive treatment effect was observed starting at Month 6 and lasting until Month 18 using autofluorescence and color fundus photographs. It appears there was no significant change in rate of atrophy extension in the bimonthly group. When stratified by size of the initial geographic area (greater or less than 10 mm2), there was a trend toward less progression in the treatment group; however, it did not appear statistically significant per initial study reports.32
When a subgroup analysis was performed on patients who were noted to be positive for exploratory biomarkers (mutations for CFH, C3, C2/CFB, and complement factor I), the treatment group was found to have a 44% reduction at the study’s conclusion compared to the sham group (P<0.005 with N=28 at the final time point). The authors note that 57% of patients assayed were positive for exploratory biomarkers. This subgroup analysis included 14 patients from the sham group and 17 from the monthly treatment group. A separate subgroup analysis of those patients in the sham and monthly treatment groups with biomarkers and BCVA 20/50–20/100 also showed a reduction in atrophic progression; however, there were only seven patients in the sham group and only ten in the treatment group for this subgroup analysis. Within the pooled sham group, the complement factor I mutation group had significantly more atrophy than the factor I negative group. The study authors conclude that the treatment response was magnified in patients with the complement factor I biomarker, which acts downstream of factor D and CFH in the alternative complement pathway.
When analyzing all patients, final BCVA appeared worse in all groups compared to baseline. The authors concluded that as vision was similarly worse in the sham versus the treatment groups, the drug itself was not implicated as a cause of acuity changes.
Safety
The MAHALO adverse events are shown in Table 5. Comparing the pooled sham group with the monthly and bimonthly groups, there was a single adverse event in the sham group study eye and three adverse events in the study eyes in the bimonthly group. There was a single event in the fellow eye in the sham group and two events in the bimonthly group. There were 15 systemic, nonocular adverse events in the sham group, eleven in the monthly group, and ten in the bimonthly group. There were four cases of adverse event in the study eye suspected to be caused by the study drug in the monthly group and three such cases in the bimonthly group. There was a single nonocular adverse event suspected to be caused by the study drug in both the monthly and bimonthly groups. There were no intraocular infections, no deaths, and no events that prompted treatment discontinuation. Conjunctival hemorrhage and eye pain were the leading adverse events in this study.
  | Table 5 MAHALO Phase II adverse events |
Conclusion/discussion
The MAHALO study suggests that monthly lampalizumab decreased GA compared with monthly sham treatment. A small subgroup analysis showed greater reduction in patients with mutations in complement factor I alleles, and in patients with moderate Snellen acuity. However, the study was not powered to look at these differences, and they remain to be tested in larger populations. In this population, a large percentage of patients were positive for complement factor I, a factor that works downstream of factor D and CFH in the alternative pathway.26
An international Phase III trial is underway to determine if lampalizumab is effective and safe in halting progression of GA in a larger population. Of the many treatments devised to treat non-neovascular AMD, lampalizumab (Genentech/Roche) is the first to have shown any promise at limiting the march of GA. Time will tell if this treatment strategy will prove effective as we await the results of the Phase III trial.
Disclosure
Dr Do is a consultant for Genentech, Regeneron, and Allergan, and has received grants from each. Dr Rhoades and Mr Dickson have no relevant financial disclosures. The authors report no other conflicts of interest in this work.
References
Congdon N, O’Colmain B, Klaver CC, et al; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. | ||
Bressler NM, Bressler SB, Congdon NG, et al; Age-Related Eye Disease Study Research Group. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003;121(11):1621–1624. | ||
Lindblad AS, Lloyd PC, Clemons TE, et al; Age-Related Eye Disease Study Research Group. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127(9):1168–1174. | ||
Brown DM, Kaiser PK, Michels M, et al; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. | ||
Rosenfeld PJ, Brown DM, Heier JS, et al; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. | ||
Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145(2):239–248. | ||
Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014. | ||
Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. | ||
Chew EY, Clemons T, SanGiovanni JP, et al; AREDS2 Research Group. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. 2012;119(11):2282–2289. | ||
Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104(10):1677–1691. | ||
Sunness JS, Applegate CA. Long-term follow-up of fixation patterns in eyes with central scotomas from geographic atrophy that is associated with age-related macular degeneration. Am J Ophthalmol. 2005;140(6):1085–1093. | ||
Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114(2):271–277. | ||
Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151–171. | ||
Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. | ||
Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol. 2014;61(2):118–125. | ||
Clark SJ, Perveen R, Hakobyan S, et al. Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch’s membrane in human retina. J Biol Chem. 2010; 285(39):30192–30202. | ||
Sofat R, Casas JP, Webster AR, et al. Complement factor H genetic variant and age-related macular degeneration: effect size, modifiers and relationship to disease subtype. Int J Epidemiol. 2012;41(1):250–262. | ||
Fuse N, Mengkegale M, Miyazawa A, et al. Polymorphisms in ARMS2 (LOC387715) and LOXL1genes in the Japanese with age-related macular degeneration. Am J Ophthalmol. 151(3):550–556. | ||
Cheng Y, Huang L, Li X, Zhou P, Zeng W, Zhang C. Genetic and functional dissection of ARMS2 in age-related macular degeneration and polypoidal choroidal vasculopathy. PLoS One. 2013;8(1):e53665. | ||
Schubert HD, et al. Retina and vitreous. In: Basic and Clinical Science Course. American Academy of Ophthalmology, 2013–2014:64. | ||
Andreoli TE. Cecil’s Essentials of Medicine, ed. Thomas E Andreoli et al. Philadelphia: Saunders, 2004:804–807. | ||
Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14(7):835–846. | ||
Charbel Issa P, Chong NV, Scholl HP. The significance of the complement system for the pathogenesis of age-related macular degeneration – current evidence and translation into clinical application. Graefes Arch Clin Exp Ophthalmol. 2011;249(2):163–174. | ||
Caire J, Recalde S, Velazquez-Villoria A, et al. Growth of geographic atropy on fundus autofluorescence and polymorphisms of CFH, CFB, C3, FHR1-3, and ARMS2 in age-related macular degeneration. JAMA Ophthalmol. 2014;132(5):528–534. | ||
Weber BH, Charbel Issa P, Pauly D, et al. The role of the complement system in age-related macular degeneration. Dtsch Arztebl Int. 2014; 111(8):133–138. | ||
Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079–1091. | ||
Yehoshua Z, Garcia Filho CAA, Gregori G, et al. Systemic complement inhibition with eculizumab for the treatment of GA in AMD patients: the COMPLETE study. Presented at the 2012 Annual Meeting of the Association for Research in Vision and Ophthalmology [ARVO], May 6–10, 2012, Fort Lauderdale, FL, USA. Abs. 2046. | ||
Do DV, Pieramici DJ, van Lookeren Campagne M, et al; Phase Ia Investigators. A phase ia dose-escalation study of the anti-factor D monoclonal antibody fragment FCFD4514S in patients with geographic atrophy. Retina. 2014;34(2):313–320. | ||
Volanakis JE, Narayana SV. Complement factor D, a novel serine protease. Protein Sci. 1996;5(4):553–564. | ||
Zipfel PF, Lauer N, Skerka C. The role of complement in AMD. Adv Exp Med Biol. 2010;703:9–24. | ||
Stanton CM, Yates JR, den Hollander AI, et al. Complement factor D in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52(12):8828–8834. | ||
Williams DF, Yaspan B, Zhengrong L, et al. Lampalizumab (anti-factor D) in Geographic Atrophy: the MAHALO Phase II Results. Presented at the 2013 American Society of Retina Specialists (ASRS) Meeting, August 27, 2013, Toronto, ON, Canada. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

