Back to Journals » Journal of Pain Research » Volume 8
Postoperative pain management with transdermal fentanyl after forefoot surgery: a randomized, placebo-controlled study
Authors Merivirta R, Pitkanen M, Alanen J, Haapoja E, Koivisto M, Kuusniemi K
Received 17 June 2014
Accepted for publication 12 October 2014
Published 16 January 2015 Volume 2015:8 Pages 39—45
DOI https://doi.org/10.2147/JPR.S69511
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Riika Merivirta,1 Mikko Pitkänen,2 Jouko Alanen,3 Elina Haapoja,1 Mari Koivisto,4 Kristiina Kuusniemi1
1Department of Anaesthesiology, Intensive Care, Emergency Care and Pain Medicine of Turku University Hospital and University of Turku, Turku, 2Department of Anaesthesia, Hospital Orton, Invalid Foundation, Helsinki, 3Terveystalo Clinic Hospital, Helsinki, 4Department of Biostatistics, University of Turku, Turku, Finland
Background: Quality of life is decreased in patients with hallux valgus deformity, mainly because of pain. Significant improvement is usually achieved by surgery. However, postoperative pain can be moderate to severe for 2–3 days. The aim of the present study was to evaluate the use of transdermal fentanyl for postoperative pain management after forefoot surgery.
Methods: Sixty patients undergoing hallux valgus or hallux rigidus surgery were allocated to receive a patch delivering either fentanyl 12 µg/hour or placebo for postoperative pain. The consumption of rescue opioid oxycodone, the primary outcome measure, was evaluated daily until the fourth postoperative day. Total consumption of oxycodone during the study period was also assessed. Pain scores and possible adverse effects were evaluated every 6 hours during the first 24 hours and on the fourth postoperative day.
Results: The use of rescue opioid was low in both groups, the median (range) consumption of oxycodone being 10 (0–50) mg on the day of surgery (no difference between the groups, P=0.31) and 0 (0–35) mg thereafter. The total combined consumption was 10 (0–105) mg in the fentanyl group and 20 (0–70) mg in the placebo group (P=0.23). There were no statistically significant differences in pain scores or adverse effects between the groups.
Conclusion: As a part of multimodal analgesia with ibuprofen and acetaminophen, a patch delivering fentanyl 12 µg/hour did not significantly decrease the consumption of rescue opioid or pain scores after forefoot surgery.
Keywords: pain management, transdermal fentanyl, multimodal analgesia, forefoot surgery, hallux valgus, hallux rigidus
Introduction
Patients with hallux valgus deformity rate their quality of life as lower compared with the general population, mainly because of pain.1 Surgery usually produces a significant improvement in this regard.1 Most patients scheduled for forefoot surgery are suited for day case surgery, if pain and nausea, the two major complaints,2 can be prevented and taken care of. Many patients with hallux valgus express moderate to severe pain 2–3 days postoperatively.2–4 Further, according to the results of our pilot study, female patients in particular seem to be sensitive to the adverse effects of strong pain medication like opioids after forefoot surgery.
In order to decrease the need for opioids, multimodal analgesia is widely recommended.5,6 Multimodal analgesia is particularly important in the treatment of ambulatory patients, as opioids tend to increase the incidence of nausea. Furthermore, orthopedic procedures are among the most painful procedures according to studies evaluating postoperative pain in outpatients.7 A transdermal fentanyl patch, used as an off-label treatment for postoperative pain, is supposed to deliver and maintain a stable plasma drug concentration, offering reliable background analgesia8 for the 3 most painful days after hallux valgus surgery. It also offers an alternative route for opioid use in patients suffering from nausea or vomiting.
The purpose of this study was to evaluate the effectiveness of transdermal fentanyl compared with placebo, each administered together with oral nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen in the treatment of postoperative pain after hallux valgus or hallux rigidus surgery.
Materials and methods
This prospective, randomized, double-blind, placebo-controlled trial was carried out at Turku University Hospital between January 2009 and November 2010. Approval of the protocol was obtained from the ethics committee of the Hospital District of Southwest Finland and the National Agency for Medicine. The study is also registered at EudraCT and ClinicalTrials.gov. All patients signed and dated an informed consent before inclusion.
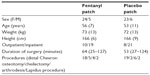
A total of 60 patients undergoing unilateral hallux valgus or hallux rigidus surgery were included. The patients, both inpatients and outpatients, were recruited from male or female patients aged 18–75 years with an American Society of Anesthesiologists physical status of I–III. Criteria for exclusion were: a previous history of intolerance to the study drug or related compounds and additives, a history of alcoholism, drug abuse, psychological or other emotional problems which might invalidate informed consent, sleep apnea and a body mass index ≥35 kg/m2.
One hour before surgery, acetaminophen 1 g (Panadol Forte®, GlaxoSmithKline, BrØndby, Denmark), was given to the patients as premedication. On the same occasion, according to randomization (using the sealed envelope technique), an opioid patch (Durogesic® 12 μg/hour; Janssen Cilag Ltd, Sollentuna, Sweden) or a placebo patch (Durogesic placebo patch, Janssen-Cilag) was placed on each patient’s skin on the upper thoracic wall. This was performed by a nurse not otherwise involved in the patient’s care. Spinal anesthesia and surgery took place in a standard fashion similar to that in cases not involved in the study. Spinal anesthesia was induced with 1.5 mL (7.5 mg) of hyperbaric bupivacaine 5.0 mg/mL (Bicain Pond Spinal® 5mg/mL, Orion, Espoo, Finland). No sedatives were used intraoperatively as a matter of routine, but if requested, propofol was administered in 10–20 mg boluses. Dexamethasone (Oradexon® 5 mg/mL, N.V. Organon, BH Oss, the Netherlands) 5 mg was given intravenously as a prophylactic antiemetic at the beginning of anesthesia. In order to prevent pain, the patients received acetaminophen 1 g and ibuprofen 600 mg (Burana®, Orion) orally in the recovery room, followed by acetaminophen 1 g and ibuprofen 600 mg three times daily for the first 4 days. In the event of severe pain, the patients received 10 mg of rapid-release oxycodone (OxyNorm®, Mundipharma, Vantaa, Finland) orally as rescue medication. Before discharge, the patients were given written instructions for removing the patch on the third postoperative day. When discharged, the patients received two 10 mg slow-release oxycodone tablets (OxyContin®, Mundipharma) to be used as rescue medication.
In mild and moderate hallux valgus cases, distal Chevron osteotomy was used. A straight medial incision was routinely performed. The medial capsule was opened in a Y-shaped manner. The medial eminence was excised and a normal V-type Chevron osteotomy was made with 5–6 mm lateral displacement of the distal fragment. In all cases, the osteotomy was fixed with one or two 1.5 mm bioabsorbable pins. Immediate weight bearing was allowed with a special postoperative shoe. In cases of severe hallux valgus, a Lapidus procedure was used. Appropriate resection of joint surfaces in the first tarsometatarsal joint was made with an oscillating saw, and correct alignment of the first metatarsal was checked with fluoroscopy. One 4.0 mm cannulated screw and a medially placed plate with unlocked and locked screws were used to achieve a sufficiently strong fixation. Postoperatively, a shoe was used as above-mentioned, with crutches and only partial weight bearing continued for 6–8 weeks postoperatively. In cases of advanced hallux rigidus, first metatarsophalangeal arthrodesis was the method of choice. A dorsal incision was used and the capsule was opened just medial to the extensor hallucis longus tendon. Hallux reamers were used to achieve round surfaces. A routine method of fixation consisted of two crossed 3.0 mm cannulated screws. Immediate weight bearing with a postoperative shoe was allowed. In cases of mild or moderate hallux rigidus, cheilectomy of the first metatarsophalangeal joint was used. All osteophytes were resected through a dorsal incision. Additionally, 20%–30% of the dorsal joint surface of the first metatarsal was resected. A small corresponding resection was made in the proximal side of the phalanx, if necessary. Peroperatively, 70–80 degrees of passive dorsiflexion was achieved in the first metatarsophalangeal joint. After the operation, weight bearing was allowed immediately and patients were encouraged to move their big toe both passively and actively as soon as tolerated.
The primary outcome measure in this study was the consumption of rescue medicine, oxycodone, and the secondary measure was pain on a numerical rating scale from 0 to 10 (0, no pain; 10, worst imaginable pain). The time points of evaluation were one hour preoperatively, immediately after surgery, at 6, 12, and 18 hours after placing the patch, and on the first and fourth postoperative days. The efficacy of pain medication in hospital was monitored by a nurse who was unaware of the patient’s treatment group at 6-hourly intervals between the surgery and the return home. Possible adverse effects, such as somnolence, nausea, itching, and obstipation, as well as breathing frequency, were also monitored using a numerical rating scale. When at home, patients were asked to register their pain scores, their worst pain, analgesic consumption, and adverse effects, using a formulated questionnaire. The patients were interviewed by the investigator during telephone follow-up on the first and/or fourth postoperative day.
The prospective power analysis showed that a sample size of 30 subjects per group would be required to show a 50% difference in rescue medication (oxycodone) use between the groups at an α-level of 0.05 and with a β-power of 0.90. The mean total oxycodone consumption of 19 mg and the standard deviation of 22 mg (on the day of surgery and the first postoperative day) were taken from our pilot study, in which the patients had a patch releasing fentanyl 12 μg/hour. We estimated that patients having a placebo patch would need twice as much oxycodone as those with a fentanyl patch, ie, 38 mg. Continuous variables were characterized in terms of the mean and standard deviation or median and range of values. In the case of categorical variables, frequencies and percentages were calculated. Differences between the treatment groups in normally distributed continuous variables (the worst pain score) were tested with the independent samples t-test. In the case of non-normally distributed variables (age, weight, height, and duration of surgery) the Mann–Whitney U test was used. Categorical variables (gender and procedures) were analyzed using the chi-square test or Fisher’s Exact test. Pain and respiratory rates were analyzed using repeated measures analysis of variance. The unstructured covariance matrix was applied. Residuals were checked for justification of the analysis, and in order to use a method that requires normally distributed residuals, square transformation was used for pain. The differences between treatment groups in the medicine being used (oxycodone) were analyzed separately at different measurement points. If there were more than three different doses of medicine being used, analyses were performed using the Mann–Whitney U test, and in the case of three or fewer doses, Fisher’s Exact test was used. P-values less than 0.05 were considered to be statistically significant. Statistical analyses were carried out using the SAS system for Windows, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Figure 1 shows a flow chart for the patients, and demographic data is presented in Table 1. The need for rescue oxycodone was low in both groups; on the day of surgery, the median dose was 10 (range 0–50) mg in both groups (P=0.30) and 0 (0–35) mg thereafter. There was no statistically significant difference in oxycodone consumption between patients with a fentanyl patch and those with a placebo patch (Table 2). The total median consumption of oxycodone during the study was 10 (0–105) mg in the fentanyl group and 20 (0–70) mg in the placebo group (P=0.23). On the day of surgery, 36% (n=10) of the patients in the fentanyl group and 25% (n=7) of those in the placebo group did not need any rescue analgesic at all (data were missing for two patients). On the first postoperative day, the proportions were 70% and 73%, respectively (n=19 in both groups).
  | Figure 1 Patient enrollment. |
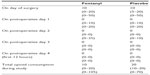  | Table 2 Rescue oxycodone use (mg) |
The interaction between group and time point in pain scores was not statistically significant (P=0.83), indicating that the groups did not differ at consecutive time points. Neither was there any statistically significant difference in pain scores (P=0.91; Figure 2). The median worst pain score during the study was 3.5 (range 0–7) on the numerical rating scale in the patients wearing a fentanyl patch and 5 (1–8) in those with a placebo patch. This difference was not statistically significant (P=0.059). The maximum scores for a single patient were 7 and 8, respectively. Many of the patients were still under the influence of spinal anesthesia when transferred to the recovery room, the median pain scores being 0 (0–4 in the placebo group and 0–5 in the fentanyl group) at that time. At 18 hours, data were available for only 12 patients in the fentanyl group and 15 in the placebo group because of the night shift.
Transdermal fentanyl did not reduce the respiratory rate. The interaction between group and time point in respiratory rate was not statistically significant (P=0.10), nor was the difference in respiratory rate (P=0.37; Figure 3A). The incidence of adverse effects did not increase. The somnolence scores are shown in Figure 3B. When asked, ten patients with a fentanyl patch and eight patients with a placebo patch reported experiencing nausea. One patient in the placebo group reported nausea spontaneously immediately after the operation. Nausea was not significant in either group, except in two patients in the fentanyl group, one of whom discontinued the study because of severe nausea. Four patients in each group had constipation on the first postoperative day. Three days later, five patients in the fentanyl group and one in the placebo group also experienced obstipation. Itching was reported by very few patients, being at its worst on the first postoperative day. Three patients in each group reported itching, rating it as 1–2 points out of 10.
Discussion
Our study showed no statistically significant differences in oxycodone consumption or pain scores after forefoot surgery between patients with a fentanyl patch and those with a placebo patch. However, total consumption of oxycodone during the study was slightly higher in the placebo group. Generally the median pain scores were lower than expected in both groups, the highest being 12 hours after placing the patch, ie, 11 hours after the beginning of surgery.
Multimodal analgesia reduces pain, opioid use, and adverse effects.5,9 A combination of preoperative dexamethasone, local anesthetic, and an NSAID during the first 3–4 postoperative days has been found to be the most favorable alternative.10 Acetaminophen is often included in a multimodal analgesia concept, but it has also been shown to be effective as monotherapy. NSAIDs are known to be effective in orthopedics, forefoot surgery included.11,12 Their use in orthopedics has been questioned because of possible impairment of bone healing,13,14 but the recent reviews are not congruent.15,16 The lack of definitive conclusions has resulted in the short-term use of NSAIDs/coxibs.3,17 In our study, patients were instructed to use ibuprofen for 4 days after surgery, and thereafter only if needed. Peripheral nerve blocks have recently gained widespread popularity, and the effects of ankle blocks and perisciatic infusions have been studied.3,18 However, by adding a single-shot ankle block to multimodal analgesia with regular etoricoxib together with additional acetaminophen and dextropropoxyphene, no significant reduction in pain or need for rescue analgesics was found during the first 24 hours postoperatively.17 As a part of multimodal analgesia, preoperative dexamethasone, given orally or intravenously, has been found to reduce both opioid consumption2 and the time to discharge.19 It also reduces the incidence of nausea,2 and is known to be an effective antiemetic.
Transdermal fentanyl is one option among the multimodal analgesics, although postoperative pain is not an official indication for its use. It may not be adequate alone, but can be considered efficient as background analgesia.8,20 The patients in our study had the worst pain at 12 hours postoperatively. The reason for this may be the residual effect of spinal anesthesia preventing pain at the time of the earlier measurements. Further, the effect of transdermal fentanyl proceeds slowly and has not reached maximum at 12 hours; in fact, a plateau of serum fentanyl concentrations was reached approximately 14 hours after placing the patch when a formulation delivering fentanyl 100 μg/hour was used.21 In another study, the mean delay time for reaching minimum effective blood fentanyl concentrations was 12.7±9.6 hours.22 Thus, the optimal time for placing the patch would be 12 hours before surgery. In the present study, because of the ambulatory nature of hallux valgus surgery, the patch could be placed only one hour before the procedure on the morning of the operation.
The effect of the fentanyl patch on postoperative pain was limited. The multimodal analgesia concept used in our patients, including the administration of NSAIDs and acetaminophen as well as dexamethasone, which has an analgesic effect, has a major effect in preventing postoperative pain.2,17 Moreover, the patch that we used, ie, delivering fentanyl 12 μg/hour, is weak. Together with the fact that the need for rescue opioid medication in both groups was very low, this may partly explain the lack of a statistically significant difference between the fentanyl and placebo groups with regard to rescue opioid consumption and pain scores. Furthermore, we considered the multimodal analgesia best for our patients, especially since most of the patients undergoing hallux surgery were women, who are known to be more sensitive to postoperative pain23 and nausea.24
As seen in our previous study,25 many patients are very self-sufficient and disregard the instructions given by hospital staff. This concerns both the amount and type of analgesics. The same observation has been made by others.26 In our study, five of 60 patients took analgesics outside the protocol, resulting in withdrawal from the study. The weakness of this study is that we were not prepared for these dropouts.
In conclusion, multimodal analgesia with regular ibuprofen and paracetamol together with a single dose of dexamethasone offered good background analgesia after surgery for hallux valgus and hallux rigidus. A patch delivering fentanyl 12 μg/hour did not reduce the need for rescue oxycodone or pain scores any further.
Disclosure
The authors report no conflicts of interests in this work.
References
Saro C, Jensen I, Lindgren U, Felländer-Tsai L. Quality-of-life outcome after hallux valgus surgery. Qual Life Res. 2007;16:731–738. | |
Mattila K, Kontinen VK, Kalso E, Hynynen MJ. Dexamethasone decreases oxycodone consumption following osteotomy of the first metatarsal bone: a randomized controlled trial in day surgery. Acta Anaesthesiol Scand. 2010;54:268–276. | |
Zaric D, Jorgensen BG, Laigaard F, Christiansen J, Burchard E. Perisciatic infusion of ropivacaine and analgesia after hallux valgus repair. Acta Anaesthesiol Scand. 2010;54:1270–1275. | |
Kim BS, Shim DS, Lee JW, Han SH, Ko YK, Park EH. Comparison of multi-drug injection versus placebo after hallux valgus surgery. Foot Ankle Int. 2011;32:856–860. | |
Elvir-Lazo OL, White PF. The role of multimodal analgesia in pain management after ambulatory surgery. Curr Opin Anaesthesiol. 2010;23:697–703. | |
Schug SA, Chong C. Pain management after ambulatory surgery. Curr Opin Anaesthesiol. 2009;22:738–743. | |
Rawal N, Hylander J, Nydahl R-A, Olofsson I, Gupta A. Survey of postoperative analgesia following ambulatory surgery. Acta Anaesthesiol Scand. 1997;41:1017–1022. | |
Lehmann LJ, DeSio JH, Radvany T, Bikhazi GB. Transdermal fentanyl in postoperative pain. Reg Anesth. 1997;22:24–28. | |
Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–1056. | |
Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: a critical assessment of the evidence. Anesthesiology. 2006;104:835–846. | |
Brattwall M, Turan I, Jakobsson J. Pain management after elective hallux valgus surgery: a prospective randomized double-blind study comparing etericoxib and tramadol. Anesth Analg. 2010;111:544–549. | |
Daniels SE, Baum DR, Clark F, Golf MH, McDonnell ME, Boesing SE. Diclofenac potassium liquid-filled soft gelatin capsules for the treatment of postbunionectomy pain. Curr Med Res Opin. 2010;26:2375–2384. | |
Dahners LE, Mullis BH. Effects of nonsteroidal anti-inflammatory drugs on bone formation and soft-tissue healing. J Am Acad Orthop Surg. 2004;12:139–143. | |
Dimmen S. Effects of cox inhibitors on bone and tendon healing. Acta Orthop Suppl. 2011;82:1–22. | |
Kurmis A, Kurmis T, O’Brien J, DalÉn T. The effect of nonsteroidal anti-inflammatory drug administration on acute phase fracture-healing: a review. J Bone Joint Surg Am. 2012;94:815–823. | |
Chen MF, Dragoo JL. The effect of nonsteroidal anti-inflammatory drugs on tissue healing. Knee Surg Sports Traumatol Arthrosc. 2013;21:540–549. | |
Turan I, Assareh H, Rolf C, Jakobsson J. Multi-modal-analgesia for pain management after hallux valgus surgery: a prospective randomised study on the effect of ankle block. J Orthop Surg Res. 2007;18:26. | |
Palmisani S, Arcioni R, Di Benedetto P, De Blasi RA, Mercieri M, Ronconi P. Ropivacaine and levobupivacaine for bilateral selective ankle block in patients undergoing hallux valgus repair. Acta Anaesthesiol Scand. 2008;52:841–844. | |
Coloma M, Duffy LL, White PF, Tongier WK, Huber PJ. Dexamethasone facilitates discharge after outpatient anorectal surgery. Anesth Analg. 2001;92:85–88. | |
Siafaka I, Rellia P, Argyra E, Iakovidou N, Sykiotis C, Vadalouka A. Pharmacokinetic profile and efficacy of a fentanyl transdermal delivery system for acute postoperative pain after intra-abdominal gynecologic surgery for cancer. Pain Pract. 2004;4:98–104. | |
Varvel JR, Shafer SL, Hwang SS, Coen PA, Stanski DR. Absorption characteristics of transdermally administered fentanyl. Anesthesiology. 1989;70:928–934. | |
Gourlay GK, Kowalski SR, Plummer JL, Cherry DA, Gaukroger P, Cousins MJ. The transdermal administration of fentanyl in the treatment of postoperative pain: pharmacokinetics and pharmacodynamic effects. Pain. 1989;37:193–202. | |
Turan I, Assareh H, Rolf C, Jakobsson J. Etoricoxib, paracetamol, and dextropropoxyphene for postoperative pain management: a questionnaire survey of consumption of take-home medication after elective hallux valgus surgery. Foot Ankle Spec. 2008;1:88–92. | |
Gan TJ, Meyer TA, Apfel CC. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615–1628. | |
Merivirta R, Kuusniemi K, Aantaa R, Hurme S, äärimaa V, Leino K. The analgesic effect of continuous subacromial bupivacaine infusion after arthroscopic shoulder surgery: a randomized controlled trial. Acta Anaesthesiol Scand. 2012;56:210–216. | |
Wilson AT, Nicholson E, Burton L, Wild C. Analgesia for day-case shoulder surgery. Br J Anaesth. 2004;92:414–415. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.