Back to Journals » Clinical Epidemiology » Volume 6
Physician practicing preferences for conventional or homeopathic medicines in elderly subjects with musculoskeletal disorders in the EPI3-MSD cohort
Authors Danno K, Joubert C, Duru G, Vetel J
Received 13 March 2014
Accepted for publication 22 May 2014
Published 26 September 2014 Volume 2014:6 Pages 333—341
DOI https://doi.org/10.2147/CLEP.S64049
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Karine Danno,1 Clementine Joubert,1 Gerard Duru,2 Jean-Marie Vetel3
1Laboratoires Boiron, Messimy, France; 2Cyklad Group, Lyon, France; 3Centre Hospitalier du Mans, Le Mans, France
Background: Musculoskeletal pain is common in elderly persons. Analgesic use is high in the elderly and may involve unacceptable risk in individuals with chronic pain. Our aim was to compare the socio-demographic characteristics of elderly subjects with musculoskeletal disorders (MSD) and to assess medication use and clinical evolution of musculoskeletal pain according to physician prescribing preference: homeopathy (Ho) group, conventional medicine (CM) group, or mixed prescription (MX) group.
Methods: The EPI3 study was a 1 year observational survey carried out among general practitioners in France between March 2007 and July 2008. This sub-analysis was carried out on elderly subjects aged ≥70 years from the original EPI3 cohort. Socio-demographic data were collected at inclusion using a self-administered patient questionnaire and medical data were recorded for each patient. Quality of life was measured using the Short Form-12 questionnaire. Patients completed a structured telephone interview on their functional status (evaluated with the QuickDash questionnaire, EIFEL scale or Lequesne index) within 72 hours of inclusion. This telephone interview was repeated at 1, 3, and 12 months. Drug exposure was also assessed during these interviews.
Results: 146 patients (mean age ± standard deviation: 75.8±4.8 years) were analyzed (80.1% female, 74.7% MSD of the spine or lower limbs, 64.4% chronic MSD). Patients in the CM and MX groups were 3.7 times or 2.5 times more likely (odds ratio [OR] =3.71, 95% confidence interval [CI]: 1.12-12.30; OR =2.52, 95% CI: 1.05-6.05; respectively) to have used non-steroidal anti-inflammatory drugs (NSAIDs) than those in the Ho group. In contrast, analgesic use was comparable in the three groups (OR =1.06 [CM versus Ho], 95% CI: 0.09-12.11; OR =0.34 [MX versus Ho], 95% CI: 0.07-1.57). Overall functional score evolution was similar in the three groups over time (P=0.16).
Conclusion: NSAID use was significantly higher in elderly MSD patients consulting a conventional practice general practitioner. In contrast, analgesic use and MSD evolution were similar in the three groups. Consulting a homeopathic physician for MSD management does not appear to represent a loss of therapeutic opportunity, and decreases the use of NSAIDs.
Keywords: elderly, musculoskeletal pain, musculoskeletal disorder, analgesic, NSAID, homeopathy
Background
Musculoskeletal pain (MSP) is a serious public health problem, adversely impacting on health status and quality of life (QoL) of affected individuals and significantly augmenting health care costs. In a population-based survey carried out between 1993 and 2006, the prevalence of disabling MSP in Spanish adults ranged from 5.5%–7.3%.1 Another Spanish National Health survey reported a 1 year prevalence of neck pain of 19.5% and a 1 year prevalence of lower back pain of 19.9%, with people 31–50 years of age, 1.5 times more likely to report lower back pain than those in the 16–30 year age group.2 In Japan, the prevalence of chronic MSP was 15.4% and was highest in subjects in the 30–50 year age range. The most common sites of pain were the lower back, neck, shoulder, and knee.3 Chronic MSP is more common in women than in men1,2 and is related to a number of factors, including low income/unemployment, accidents in the previous year, low educational level, age 45–64 years (in women only), obesity, sedentary lifestyle, and presence of comorbid chronic diseases.1
The prevalence of MSP is particularly high in older subjects. In a community-based survey of 5,093 ambulatory subjects aged ≥65 years in the USA, 42% reported MSP in the first year of the study, 32% reported chronic pain lasting for 3 or more years and 32% reported intermittent pain.4 MSP in elderly persons is frequently associated with depression,4–6 a risk of falls,6,7 sleep difficulty,8 mobility limitation,5,9–11 and poor QoL.12 The risk of disability increases with the number of sites affected by MSP.13
Analgesics are the most common drugs used by community-dwelling adults ≥75 years of age, and pain in the previous month is reported by over 70% of these analgesic users.14 Despite taking analgesics, however, many elderly subjects remain in pain.3,15 Thus, the appropriateness of this medication in these individuals is questionable. Furthermore, elderly subjects often receive polymedication for several comorbidities and may take analgesic drugs for pain relief along with ten or more other non-analgesic medicines. Polymedication use is commonly associated with the appearance of iatrogenic adverse events and drug interactions, which are directly related to the number of drugs being taken.16 Iatrogenic side effects are responsible for 10% of hospital admissions in individuals >65 years of age and >20% in subjects aged >80 years.17 Cardiovascular drugs, anti-vitamin K, psychotropics, anticoagulants, and non-steroidal anti-inflammatory drugs (NSAIDs) are the main drugs associated with side effects.18,19 An effective solution is therefore required to prevent inappropriate drug use whilst managing MSP in the elderly population.
In this report, we describe a sub-analysis of the data from the EPI3 general practice survey carried out previously in France.20,21 An earlier report from this study described the effect of physician practicing preferences (PPP) on exposure to NSAIDs in adults with musculoskeletal disorders (MSD) in primary care.21 This sub-analysis was carried out on a sub-population of the original EPI3-MSD cohort. Our aim was to compare the socio-demographic and clinical characteristics of elderly subjects ≥70 years of age consulting general practitioners (GPs) with three types of prescribing preference: homeopathy (Ho) only group; mixed prescription (MX) group, patients consulting physicians prescribing conventional plus homeopathic medicines; and conventional medicine (CM) only group; and to assess the therapeutic management of these subjects in terms of medication use and clinical evolution of MSP.
Methods
Study design and participants
The EPI3 study was a 1 year observational survey carried out among a representative sample of GPs and their patients in France between March 2007 and July 2008.20,21
The study participants were selected by two-stage sampling, as described previously.20,21 Briefly, GPs, randomly selected from the French national directory of physicians, were invited to participate. Recruitment was stratified according to PPP, which was self-declared by the GPs and categorized into three groups: i) physicians who prescribed strictly conventional medicine (CM group); these GPs declared that they never or rarely use homeopathy or complementary and alternative medicines (CAM); ii) physicians who used CAM regularly in a mixed practice (MX group); and iii) registered homeopathic GPs (Ho group) who mainly prescribed homeopathic medicines. Participating physicians completed a short telephone questionnaire allocating them to one of these three categories. GP selection continued until the sampling ratios reached 2:1 and 3:2 for the MX and Ho groups, respectively, relative to the CM group. These ratios accounted for the variety of practices, particularly in the MX group, which does not represent a single professional entity.20,21
During a second stage of sampling, one consultation day was selected for each participating GP to survey all patients attending the practice on that day.
MSD subjects in the original study were included if their health status and literacy level allowed them to complete a self-administered questionnaire. The current analysis included all elderly persons aged ≥70 years with MSD as their main reason for consultation. A functional score was available at 72 hours via a telephone interview for all subjects. MSD consisted of spinal (International Classification of Diseases [ICD] codes: 720–724) and non-spinal (ICD codes: 715, 719, 729, 726–728, and 782) disorders.
The study was approved by the French National Data-Protection Commission and the French National Council of Physicians. Participating physicians received monetary compensation for their time in providing the study data, but MSD patients received no financial remuneration.
Data collection
The collection of data for the original EPI3 cohort is described in detail elsewhere.20,21 Briefly, on the chosen consultation day for each practice, eligible patients completed a self-administered questionnaire including the following socio-demographic and functional data: age, sex, education, employment status, complementary insurance, hospitalization and medical visits in the previous 12 months, smoking status, alcohol intake, physical activity, height, weight, and health-related QoL assessed using the Short Form-12 (SF-12) questionnaire.22 The GPs completed a medical questionnaire for each patient listing: the main reason for consultation; up to five comorbidities present on that day; and for each comorbidity, the duration of the current episode of the problem. Diagnoses were coded by a trained archivist using the ninth revision of the ICD (ICD9).
Within 72 hours of recruitment, participating MSD patients received a telephone call and were asked to complete a structured interview on their current functional status. The interview consisted of the French adaptation of the Roland-Morris questionnaire (EIFEL) for back pain, the QuickDash for MSP of the upper limbs and the Lequesne for MSP of the lower limbs.23–25 This telephone interview was repeated at 1, 3, and 12 months. Drug exposure was assessed during the interviews using a standardized method known as Progressive Assisted Backward Active Recall.26 Briefly, at inclusion in the study, patients were given a booklet describing the interview and including a list of commonly used drugs for MSD. The reference period for drug exposure was the previous month at the 1 month interview, and the previous 2 months at the 3 and 12 month interviews. All medications for MSD listed by the patients were entered into a database that automatically assigned them their corresponding Anatomical Therapeutic Chemical (ATC) codes, revision 2009.
Loss of therapeutic opportunity for elderly MSD patients was assessed during the 12 month follow-up period. This was defined as progression from a non-chronic MSD at inclusion to a chronic MSD and by the development of anxiety/depression, defined as the start of psychotropic treatment during the 12 month follow-up period.
Statistical analyses
MSD progression was determined by measuring functional scores at 1, 3, and 12 months; these were then compared to the score at the 72 hours interview (baseline value). Functional scores were standardized at 100 points from their original scales. The higher the standardized functional score, the greater the functional disability. Changes in functional score were categorized for each individual as: improved = 12 month functional score 12.5 points (standardized over 100) higher than baseline value; or not improved = functional score otherwise.27 The proportion of improved patients was compared in the three groups by multiple logistic regression analysis, adjusting for baseline functional score, PPP (CM, MX, and Ho), follow-up time, sex, physician role (regular physician or not), and SF-12 score at inclusion.
Exposure of elderly MSD patients to NSAIDs (ATC codes starting with M01A [anti-inflammatory and antirheumatic products, non-steroidal]) and analgesics (ATC codes starting with N02A [opioids] and N02B [other analgesics and antipyretics]) in the MX and CM groups was compared to exposure in the Ho group. Drug exposure was first dichotomized as exposed or not exposed at least once during any of the three time intervals, and groups were further compared using logistic regression analysis adjusting for baseline functional score, sex, and SF-12 scores at inclusion. The physician’s role (regular physician or not) could not be incorporated in this model.
All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of the study population
Of the 8,559 patients recruited to the EPI3 cross-sectional study, 1,840 (21.5%) were adults consulting mainly for an MSD, of whom 1,143 (62.7%) patients recruited by 825 GPs across France consented to participate, comprising the EPI3-MSD study database.21 From this database, the current analysis included 146 patients aged ≥70 years (mean age: 75.8±4.8 years) recruited by 119 GPs. The composition of the three groups of patients according to PPP is shown in Figure 1. The socio-demographic and clinical characteristics of the patients according to PPP are summarized in Table 1. Over three quarters (80.1%) of the patients were female, 74.7% had an MSD localized to the spine or lower limbs and 64.4% had a chronic MSD.
  | Figure 1 Composition of the three groups of patients. |
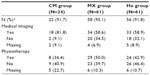
There was no significant difference in the socio-demographic characteristics (sex, age), alcohol consumption, hospitalization, medical imaging, and physiotherapy in the previous year, MSD (diagnosis and chronicity) and QoL (evaluated with the SF-12) between the three groups of patients at inclusion (Table 1). However, the patients in the three groups differed significantly according to their smoking history, status of GP consulted (regular physician or not) and their functional capacity at 72 hours (evaluated with the QuickDash questionnaire, EIFEL scale or Lequesne index). The Ho group contained significantly more patients who had never smoked than the CM group (78.7% versus [vs] 50.0%, respectively; P=0.034). Patients who consulted a conventional-medicine or mixed-prescribing physician (CM or MX groups) declared more often that this physician was their regular GP, compared with those who consulted a homeopathic physician (Ho group) (79.2% and 82.0% vs 52.2%, respectively; P<0.001). Finally, patients in the CM group had a statistically higher functional score (49.6±21.7) (ie, greater functional disability) at inclusion than patients in the Ho group (35.8±19.3) (P=0.008) or MX group (37.2±22.9) (P=0.017).
Over 65% of patients in each group had a comorbidity at inclusion. The most frequent comorbidities in all three groups were circulatory problems (Table 1).
Treatments prescribed at inclusion
The content of the prescriptions given to the elderly MSD patients at inclusion is coherent with the PPP for the three groups. No patients in the CM group were prescribed a homeopathic medicine vs 15.3% in the MX group and 74.6% in the Ho group (Table 1). Conversely, patients in the Ho group were prescribed conventional medicines less often than those in the CM group (59.3% vs 100% of prescriptions, respectively) and were more often prescribed medicines such as vitamins, minerals, and trace-elements (32.2% vs 8.7%) (Table 1). Patients in the CM group were three times more likely to be prescribed analgesics and NSAIDs than those in the Ho group.
Evolution of MSD
The level of long-term participation in this study was satisfactory and only 15.8% of patients were lost to telephone follow-up at 12 months. Figure 2 shows the evolution of MSD according to the standardized functional scores for each group over the 12 month follow-up period. A decrease in functional score at 1 month was observed in the Ho group (mean score 37.5 at 72 hours and 27.1 at 1 month) but not in the other two groups. However, analysis of covariance (ANCOVA) for repeated measures showed no significant difference in evolution of functional scores between the three groups over time (P=0.16).
  | Figure 2 Evolution of the functional score of the three groups of MSD patients (N=99), as a function of prescribing practice of the physician consulted at inclusion. |
In contrast, for individual scores, a higher proportion of patients in the Ho group improved over the 12 month follow-up period compared with the CM and MX groups (40.0% vs 22.2% vs 13.0%, respectively) (data not shown).
Medication use during the 12 month study
At end of study, the probability of having used analgesics during the 12 months was comparable in the three groups of patients (odds ratio [OR] =1.06 [CM vs Ho] [95% confidence interval (CI): 0.09–12.11]); OR =0.34 (MX vs Ho [95% CI: 0.07–1.57]) (Table 2). Overall, 95.5% of patients in the CM group declared that they had used analgesics vs 87.5% in the Ho group and 84.5% in the MX group.
In contrast, patients in the CM group were 3.7 times more likely (OR =3.71 [95% CI: 1.12–12.30]), and patients in the MX group were 2.5 times more likely (OR =2.52 [95% CI: 1.05–6.05]), to have used NSAIDs than those in the Ho group (Table 2). At the 12 month follow-up, 63.6% of patients in the CM group declared that they had used NSAIDs vs 28.6% in the Ho group and 50.0% in the MX group.
At the 12 month follow-up, 22.7% and 20.7% of patients in the CM and MX groups, respectively, stated that they had used homeopathic medicines vs 85.7% of patients in the Ho group (Table 2).
Medical imaging was used more often during the 12 month follow-up period in the CM group (81.8%) than in the MX (58.6%) and Ho (58.9%) groups (Table 3). In contrast, patients in the MX group (50.0%) and Ho group (42.9%) were more likely to have had physiotherapy than those in the CM group (36.4%) (Table 3).
Among the 123 patients who took part in an end of study interview and who did not take psychotropic drugs at inclusion, 13 patients (10.6%) had used psychotropic medications during the follow-up period. It is not possible to carry out a statistical evaluation of the association between PPP (CM, MX or Ho) and use of psychotropics, however, due to the small number of events recorded.
Discussion
To our knowledge, this sub-analysis of the EPI3-MSD cohort is the first to provide comparative data on the management of MSD in elderly subjects aged ≥70 years according to PPP. Our results show that elderly subjects who consulted a conventional prescribing-practice physician at inclusion used more NSAIDs than those who consulted a homeopathic or mixed prescribing-practice physician. Elderly patients who consulted a conventional-medicine physician were 3.7 times (OR =3.71 [95% CI: 1.12–12.3]) more likely to be prescribed NSAIDs than those who consulted a homeopathic physician. This increased prescription of NSAIDs by conventional-medicine physicians is most likely due to the GPs’ prescribing preference, but could also be due to the fact that patients consulting a conventional prescribing-practice physician had higher functional scores and therefore a greater level of disability at inclusion than those consulting mixed prescribing-practice or homeopathic physicians. Whatever the reason, the difference in NSAID use did not appear to affect the long-term outcome of these elderly MSD patients, because the evolution of functional scores over the 12 months was statistically similar in the three treatment groups (CM, MX, and Ho).
In contrast to the use of NSAIDs, and despite the fact that significantly more patients in the CM group were prescribed analgesics at inclusion than in the other two groups, analgesic use over the 12 month study was comparable in the three groups of patients (87.5% of patients used analgesics during follow-up), presumably because many patients purchased these drugs over-the-counter or already had them at home in their medicine cabinets. It is noteworthy that functional scores did not differ significantly between inclusion and at the 12 month follow-up in any of our three treatment groups, which is coherent with previous observations that many elderly subjects remain in pain and continue to have significant functional disability despite taking analgesics.3,11,15
Elderly subjects frequently receive polymedication, defined as the concurrent use of five or more drugs.28 It has been reported that 40% of elderly subjects in the USA use polymedication, while 12% of persons aged ≥65 years take ten or more drugs on a daily basis.28,29 Similar levels of use have been reported in Europe.30 The problems associated with polymedication in the elderly, including altered drug action, adverse reactions, and adverse interactions, have been well documented.31,32 The risk of side effects and drug interactions is directly related to the number of drugs being taken.16 Prybys et al showed that the risk of an adverse drug reaction is 60% in subjects who take five or more drugs and 80% in those who take seven or more.33 Furthermore, adverse drug reactions were the fifth leading cause of death in the USA in 1998.34 Many of the drugs taken by elderly patients are inappropriate. An update of the Beers criteria for potentially inappropriate medication use in older adults recommends 48 individual medications or classes of medications to be avoided in older adults, and 20 medications to be avoided in older adults with specific conditions. Sixty-six of these potentially inappropriate drugs are considered to have side effects of high severity.35 Management of mild to moderate pain is traditionally based on the use of NSAIDs or the analgesic, paracetamol. NSAIDs are often poorly tolerated due to gastrointestinal side effects and may in severe cases cause peptic ulcers, perforation, and bleeding.36,37 NSAIDs are not recommended in elderly subjects with gastric or duodenal ulcers35 and current guidelines focus on paracetamol as the first-line choice for the management of chronic MSP.38 However, our results suggest that analgesics may also be inappropriate, because they do not appear to bring about long-term relief in many elderly patients with MSP.
Some authors have recommended that drug treatment should be avoided in the elderly, if possible, and that complementary and alternative approaches should be tried first for non-acute conditions.31,32 Cognitive behavioral therapy, spinal manipulation, mobilization, and exercise all have moderate efficacy for the treatment of chronic or subacute lower back pain and neck pain.39,40 In a more recent study of elderly patients aged ≥75 years, a comprehensive geriatric assessment and individually tailored multifactorial intervention including physical activity counselling and supervised resistance training had a positive effect on mobility and MSP.41 The integration of CAM, including homeopathy, into conventional medical practice for chronic and subacute conditions such as MSP could greatly reduce the use of some medications and the prevalence of adverse reactions associated with polymedication.28
Strengths and limitations
Our study has a number of strengths. Firstly, MSD patients were identified from a large sample of patients consulting for any reason in primary care, meaning the study was more likely to be representative of a real-life population. Patients were followed and their MSD was assessed over a long period of time (12 months). Drug exposure was obtained from patient interviews using a previously validated method,26 although the assessment period only covered 5 months of the 12 month study, and therefore, true exposure was likely to have been underestimated. However, the fact that the same method of assessment was used for all patients reduces the possibility of bias in the estimation of exposures. Combining medical information on diagnoses with patient-assessed information on drug utilization, obtained using a validated questionnaire,26 provided an additional level of control for the quality of the study data.
The study is limited by the fact that only restricted measures (MSD functional scale) were included to assess MSD evolution according to PPP. Furthermore, the three treatment groups were not comparable in terms of size and severity of MSD at inclusion. The CM group was smaller than the other two groups (MX and Ho) and functional disability linked to MSD was greater in the CM group at inclusion. Moreover, those elderly patients who required a home visit from their GP were not included in the analysis. Thus, it is possible that prescriptions may vary according to functional severity as well as PPP. Finally, the proportion of patients taking analgesics in each group was similar, and therefore, patients in the Ho group were not strictly homeopathy users only. This is difficult to control for in a longitudinal survey, because analgesics are freely available over-the-counter and do not require a medical prescription. Nevertheless, the lack of any statistically significant improvement in MSP over the 12 months shows that analgesics are frequently ineffective and could be replaced by other types of pain or mobility control. In this case, consulting a homeopathic physician for MSD management does not seem to represent a loss of therapeutic opportunity and could reduce the use of NSAIDs in elderly subjects.
Conclusion
The use of homeopathic medicines for MSP was associated with the decreased use of NSAIDs. NSAID use was significantly higher in elderly patients consulting a conventional prescribing-practice physician than in those consulting a mixed prescribing-practice or homeopathic physician. Patients in the CM group were 3.7 times (OR =3.71 [95% CI: 1.12–12.3]) more likely to use NSAIDs than those in the Ho group. In contrast, the use of analgesics by elderly MSD patients was similar in the three groups (CM, MX, and Ho). Evolution of functional scores of MSD was also similar in the three groups of elderly patients. Consulting a homeopathic physician for MSD management does not seem to represent a loss of therapeutic opportunity.
Author contributions
The analyses were conceived by JMV. CJ was responsible for data collection and statistical analysis, with assistance from GD. All authors contributed to the interpretation of the data. KD wrote the first draft of the article and all authors contributed to its further development. All authors read and approved the final manuscript.
Acknowledgment
The authors thank Newmed Publishing Services for providing professional writing support, funded by Laboratoires Boiron.
Disclosure
Laboratoires Boiron provided financial support for the study. KD and CJ are employees of Laboratoires Boiron. The remaining authors have no conflicts of interest to disclose.
References
Jiménez-Sánchez S, Jiménez-García R, Herrnández-Barrera V, Villanueva-Martínez M, Ríos-Luna A, Fernández-de-las-Peñas C. Has the prevalence of invalidating musculoskeletal pain changed over the last 15 years (1993–2006)? A Spanish population-based survey. J Pain. 2010;11(7):612–620. | |
Fernández-de-las-Peñas C, Herrnández-Barrera V, Alonso-Blanco C, et al. Prevalence of neck and low back pain in community-dwelling adults in Spain. Spine. 2011;36(3):E213–E219. | |
Nakamura M, Nishiwaki Y, Ushida T, Toyama Y. Prevalence and characteristics of chronic musculoskeletal pain in Japan. J Orthop Sci. 2011;16(4):424–432. | |
Thielke SM, Whitson H, Diehr P, et al. Persistence and remission of musculoskeletal pain in community-dwelling older adults: results from the cardiovascular health study. J Am Geriatr Soc. 2012;60(8):1393–1400. | |
Reid MC, Williams CS, Gill TM. The relationship between psychological factors and disabling musculoskeletal pain in community-dwelling older persons. J Am Geriatr Soc. 2003;51(8):1092–1098. | |
Eggermont LH, Penninx BW, Jones RN, Leveille SG. Depressive symptoms, chronic pain, and falls in older community-dwelling adults: the MOBILIZE Boston study. J Am Geriatr Soc. 2012;60(2):230–237. | |
Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302(20):2214–2221. | |
Chen Q, Hayman LL, Shmerling RH, Bean JF, Leveille SG. Characteristics of chronic pain associated with sleep difficulty in older adults: the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly (MOBILIZE) Boston study. J Am Geriatr Soc. 2011;59(8):1385–1392. | |
Mottram S, Peat G, Thomas E, Wilkie R, Croft P. Patterns of pain and mobility limitation in older people: cross-sectional findings from a population survey of 18,497 adults aged 50 years and over. Qual Life Res. 2008;17(4):529–539. | |
Shah RC, Buchman AS, Boyle PA, et al. Musculoskeletal pain is associated with incident mobility disability in community-dwelling elders. J Gerontol A Biol Sci Mec Sci. 2011;66(1):82–88. | |
Karttunen N, Lihavainen K, Sipila S, Rantanen T, Sulkava R, Hartikainen S. Musculoskeletal pain and use of analgesics in relation to mobility limitation among community-dwelling persons aged 75 years and older. Eur J Pain. 2012;16(1):140–149. | |
Laslett LL, Quinn SJ, Winzenberg TM, Sanderson K, Cicuttini F, Jones G. A prospective study of the impact of musculoskeletal pain and radiographic and osteoarthritis on health related quality of life in community dwelling older people. BMC Musculoskelet Disord. 2012;13:168. | |
Buchman AS, Shah RC, Leurgans SE, Boyle PA, Wilson RS, Bennett DA. Musculoskeletal pain and incident disability in community-dwelling older adults. Arthritis Care Res (Hoboken). 2010;62(9):1287–1293. | |
Pokela N, Bell JS, Lihavainen K, Sulkava R, Hartikainen S. Analgesic use among community-dwelling people aged 75 years and older: a population-based interview study. Am J Geriatr Pharmacother. 2010;8(3):233–244. | |
Sawyer P, Bodner EV, Ritchie CS, Allman RM. Pain and pain medication use in community-dwelling older adults. Am J Geriatr Pharmacother. 2006;4(4):316–324. | |
Hobson M. Medications in older patients. West J Med. 1992;157(5):539–543. | |
Derrien E. Risque iatrogène chez la personne âgée [Iatrogenic risk in the elderly]. Revue du Soignant en Geriatrie. 2004;12:15–21. French. | |
Doucet J, Queneau P. Effets indésirables des médicaments chez les sujets âgés: Accidents médicamenteux: comment les prévenir. [Adverse drug reactions in the elderly]. Bull Acad Natl Med. 2005;189:1693–1709. French. | |
Stoianovici I, Orru E, et al. Étude d’évitabilité sur 130 cas d’accidents iatrogéniques déclarés en pharmacovigilance dans un service de gériatrie [Preventability study of 130 cases of iatrogenic accidents reported in pharmacovigilance within a Geriatrics Department]. Rev Geriatrie. 2008;33:277–284. French. | |
Grimaldi-Bensouda L, Bengaud B, Lert F, et al. Benchmarking the burden of 100 diseases: results of a nationwide representative survey within general practices. BMJ Open. 2011;1(2):e000215. | |
Rossignol M, Bergaud B, Engel P, et al. Impact of physician preferences for homeopathic or conventional medicines on patients with musculoskeletal disorders: results from the EPI3-MSD cohort. Pharmacoepidemiol Drug Saf. 2012;21(10):1093–1101. | |
Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171–1178. | |
Coste J, Le Parc JM, Berge E, Delecoeuillerie G, Paolaggi JB. Validation française d’une échelle d’incapacité fonctionnelle pour l’évaluation des lombalgies (EIFEL) [French validation of a disability rating scale for the evaluation of low back pain (EIFEL questionnaire)]. Rev Rhum Ed Fr. 1993;60(5):335–341. French. | |
Beaton DE, Wright JG, Katz JN; Upper Extremity Collaborative Group. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87(5):1038–1046. | |
Lequesne MG, Méry C, Samson M, Marty M. Comparison between the WOMAC and the Lequesne indices in patients with knee and hip osteoarthritis. Osteoarthritis Cartilage. 1998,6(6):441–442. | |
Grimaldi-Bensouda L, Rossignol M, Aubrun E, El Kerri N, Benichou J, Abenhaim L, PGRx Study Group. Agreement between patients’ self-report and physicians’ prescriptions on cardiovascular drug exposure: the PGRx database experience. Pharmacoepidemiol Drug Saf. 2010;19(6):591–595. | |
Maughan EF, Lewis JS. Outcome measures in chronic low back pain. Eur Spine J. 2010;19(9):1484–1494. | |
Jacobs J, Fisher P. Polypharmacy, multimorbidity and the value of integrative medicine in public health. Eur J Integr Med. 2013;5(1):4–7. | |
Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337–344. | |
Fialova D, Topinkova E, Gambassi G, et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA. 2005;293(11):1348–1358. | |
Oh VM. Multiple medications: problems of the elderly patient. Int Dent J. 1991;41(6):348–358. | |
Brawn LA, Castleden CM. Adverse drug reactions. An overview of special considerations in the management of the elderly patient. Drug Saf. 1990;5(6):421–435. | |
Prybys K, Melville K, Hanna J, Gee A, Chyka P: Polypharmacy in the elderly: clinical challenges in emergency practice: Part 1: Overview, etiology, and drug interactions. Emergency Medicine Reports. 2002;23:145–153. | |
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–1204. | |
Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–2724. | |
Langford RM. Pain management today – what have we learned? Clin Rheumatol. 2006;25(Suppl 1):S2–S8. | |
Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig. 2012;32(Suppl 1):53–63. | |
Schnitzer TJ. Update on guidelines for the treatment of chronic musculoskeletal pain. Clin Rheumatol. 2006;25(Suppl 1):S22–S29. | |
Bronfort G, Haas M, Evans RL, Bouter LM. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: a systematic review and best evidence synthesis. Spine J. 2004;4(3):335–356. | |
Chou R, Huffman LH; American Pain Society; American College of Physicians. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):492–504. | |
Lihavainen K, Sipilä S, Rantanen T, Kauppinen M, Sulkava R, Hartikainen S. Effects of comprehensive geriatric assessment and targeted intervention on mobility in persons aged 75 years and over: a randomized controlled trial. Clin Rehabil. 2012;26(4):314–326. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.