Back to Journals » Patient Preference and Adherence » Volume 9
Patient-reported outcomes and considerations in the management of COPD: focus on aclidinium
Authors Lopez-Campos JL , Calero C, Lopez-Ramirez C, Asensio-Cruz MI, Marquez-Martín E, Ortega F
Received 27 September 2014
Accepted for publication 12 November 2014
Published 17 January 2015 Volume 2015:9 Pages 95—104
DOI https://doi.org/10.2147/PPA.S55009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Jose Luis Lopez-Campos,1,2 Carmen Calero,1,2 Cecilia Lopez-Ramirez,1 Maria Isabel Asensio-Cruz,1 Eduardo Márquez-Martín,1 Francisco Ortega-Ruiz1,2
1Unidad Médico-Quirúrgica de Enfermedades Respiratorias, Instituto de Biomedicina de Sevilla (IBiS), Hospital Universitario Virgen del Rocío/Universidad de Sevilla, Sevilla, 2CIBER de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, Spain
Abstract: Chronic obstructive pulmonary disease (COPD) is a complex heterogeneous disease, in which several factors combine to give the final clinical expression. Both early and more recent studies have shown that forced expiratory volume in one second (FEV1), despite being an extremely important parameter to predict the progression of the disease, is a poor surrogate marker for symptoms perception. Accordingly, patient-reported outcomes (PROs) have gained popularity as a measure of the impact of treatment from the patients’ perspective, since they represent the individuals’ perception of their health status, beyond any physiological limitations. Several such PROs, therefore, are currently included in multidimensional COPD evaluation. This multidimensional approach helps identify different patient types and individualize, up to a certain point, pharmacological treatment. In this multidimensional approach it is important to highlight the importance of long-acting bronchodilators in COPD treatment strategies. Long-acting bronchodilators are cost-effective and have been shown to achieve the greatest functional and clinical improvements in COPD. As a result, long-acting bronchodilators are now the main pharmacological treatment for COPD at all stages of the disease. Until recently, tiotropium was the leading bronchodilator for the treatment of COPD. The clinical development of this medication, unprecedented in inhaled therapy, involved tens of thousands of patients and yielded consistent outcomes in terms of lung function, symptoms, quality of life, exacerbations, and prognosis. However, new long-acting bronchodilators have recently been developed or are currently under development. In this review, we evaluate the effects of aclidinium bromide, a novel long-acting bronchodilator, on PROs in COPD. Aclidinium is a novel long-acting muscarinic antagonist with a good safety profile for the treatment of COPD, and has proven efficacy in both objective functional measurements and PROs. Comparison studies with tiotropium have shown it to have similar lung function improvement and a similar impact on PROs, including quality of life or symptom perception.
Keywords: patient-reported outcomes, chronic obstructive pulmonary disease, bronchodilators, aclidinium
Introduction
Although recent studies have shown a steady decrease in chronic obstructive pulmonary disease (COPD) mortality,1 it is still the third leading cause of death.2 Additionally, the impact of COPD on health-related quality of life3 and the burden on health care systems4 make it a disease of the first order.
As an obstructive disease, the main parameter to evaluate progression is the degree of airflow obstruction measured by the forced expiratory volume in one second (FEV1) obtained during a forced spirometry. As a result, traditional clinical trials have focused on demonstrating improvement in FEV1 either as an isolated measurement or as a declining trend over time.5 Accordingly, previous guidelines focused on establishing treatment strategies according to the degree of FEV1 impairment.6
However, COPD is a complex heterogeneous disease, in which several factors combine to give the final clinical expression. Both early and more recent studies have shown that, despite being an extremely important parameter to predict the progression of the disease, FEV1 is a poor surrogate marker for symptoms perception. In the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) study, a large, 3-year observational controlled multicenter international study aimed at defining clinically relevant subtypes of COPD,7 the authors very elegantly showed the complex interaction between FEV1 and clinical markers. As a result, although clinical symptoms worsen as FEV1 decreases in the cohort of patients, when it comes down to the individual patient, the authors found different degrees of impairment in symptoms or exacerbations in all FEV1-mediated COPD degrees of severity (Figure 1).8 This suggested that new markers of disease expression and progression were needed to make a correct and more comprehensive evaluation of COPD patients.
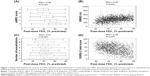  | Figure 1 Relationship between the severity of airflow limitation and breathlessness as assessed by the mMRC questionnaire (panel A), exercise capacity as assessed by the 6MWD (panel B), reported exacerbations in the year before inclusion in the study (panel C), and health status as assessed by the SGRQ-C (panel D). |
Consequently, in recent years, a shift toward patient-centered medicine has been proposed, and several initiatives have been put forward to include other clinical variables in the so-called multidimensional evaluation of the COPD patient, in which FEV1 continues to play a prominent role, but is modulated by other clinical disease expressions.9 Accordingly, since the 2011 update, the Global initiative for chronic Obstructive Lung Disease incorporates a three-pronged approach (lung function, chronic symptoms, and exacerbations) to identify types of patients who may need different treatment strategies.10 Although controversial,11 one of the strengths of this approach is the inclusion of patient-reported outcomes (PROs) as part of the evaluation system. PROs have gained popularity as a measure of the impact of treatment from the patients’ perspective, since they represent the individuals’ experience of their health status, beyond any physiological limitations.12,13 This multidimensional approach helps identify different patient types and individualize, up to a certain point, pharmacological treatment.
In this system, it is important to highlight the importance of long-acting bronchodilators in COPD treatment strategies. Long-acting bronchodilators are cost-effective14 and have been shown to achieve the greatest functional and clinical improvements in COPD.15 As a result, long-acting bronchodilators are now the main pharmacological treatment for COPD at all stages of the disease.16
Until recently, tiotropium was the leading bronchodilator for the treatment of COPD. The clinical development of this medication, unprecedented in inhaled therapy, has involved tens of thousands of patients and yielded consistent outcomes in terms of lung function, symptoms, quality of life, exacerbations, and prognosis in both the Handihaler and the Respimat presentations.17–20 Two large studies have confirmed the safety of the drug in both presentations.17,20
In recent years, new long-acting bronchodilators, including long-acting beta agonists and long-acting muscarinic antagonists (LAMAs), have been developed or are under development (Table 1), and have considerably increased the possibility of treating COPD patients with monotherapy or a combination of these drugs. It follows that clinicians may need an updated review on some of these drugs. In this review, we evaluate the effects of aclidinium bromide, a novel long-acting bronchodilator, on PROs in COPD.
  | Table 1 Inhaled drugs for asthma and COPD |
PROs in COPD
It is widely accepted that a correct multidimensional evaluation of COPD patients requires both objective and subjective measurement of their health status. Objective measurements provide a comparable means of evaluating the progression of the disease and making intra- and intersubject comparisons. In COPD, these are based on functional evaluations such as spirometry, lung volumes, diffusing capacity, or exercise capacity. Additionally, other objective nonfunctional measurements can be of help in identifying certain types of patients, including high-resolution computed tomography to identify bronchiectasis, sputum culture to identify bacterial colonization, or different tests to identify the Asthma and COPD Overlap Syndrome.21,22 However, objective measurements correlate poorly with the patient’s subjective experience, and should be complemented by subjective PRO.
The term PRO has been coined to describe the patient’s self-perceived health status.13 PROs are outcomes reported directly by patients, usually by means of self-administered questionnaires or diaries, or during a structured interview. In this way, they capture the individual’s experiences of COPD without any interpretation from third parties.
PRO, being a subjective expression based on symptoms and the perception of the disease, is difficult to measure objectively. Accordingly, multiple instruments designed to obtain an objective measurement from subjective PROs have been developed, ranging from one-dimensional symptom scales to multidimensional strategies.
The most relevant PRO instruments and scales for COPD are summarized in Table 2.12 Many of these scales or questionnaires can be divided into two different types: one-dimensional scales to evaluate particular symptoms and multidimensional questionnaires to assess health status. Among the former, dyspnea is the most prevalent symptom in COPD and has a good number of questionnaires. The modified Medical Research Council (MRC) scale initially developed by Fletcher23 is the most popular and the one recommended by current guidelines.10 However, the MRC questionnaire is not very sensitive to changes, and therefore new questionnaires have been used in clinical trials, the most popular being the Transition Dyspnea Index (TDI). Developed by Mahler et al24 it consists of two questionnaires, the Baseline Dyspnea Index that rates the severity of dyspnea at a single state and a TDI that denotes changes from baseline. TDI changes in dyspnea are divided into seven grades, ranging from −3 (major deterioration) to +3 (major improvement) and three categories. The ratings for each of the three categories were added to form a transition focal score (range from −9 to +9), where a negative score indicates deterioration and a positive score indicates improvement. The minimum clinically important difference has been studied and set at one point of the scale.25 This TDI is used in clinical trials, including those evaluating aclidinium.
  | Table 2 Principal patient-reported scales and questionnaires for COPD |
One particular PRO is physical activity. Although physical activity is considered an important therapeutic target in COPD, what “physical activity” means to COPD patients and how their perspective is best measured are poorly understood. Recently, a conceptual framework for the development and content validation of two PRO physical activity instruments (PROactive PRO instruments) has been developed.26
Other important symptoms-related PROs in COPD are chronic cough and sputum. Despite the overwhelming importance of these symptoms in several clinical outcomes such as quality of life, exacerbations, and prognosis, the development of scales or questionnaires to assess these symptoms has been much slower. The main questionnaire used is the Cough and Sputum Assessment Questionnaire, which evaluates clinical symptoms and their impact on patients with COPD or chronic bronchitis.27
With the advance of new knowledge in the understanding of the disease, new PROs are being identified. In particular, a large pan-European study recently identified symptom perception variability for COPD patients.28 This study found that, contrary to common knowledge, COPD patients experience variability in the perception of their symptoms. Most strikingly, symptoms are most frequently perceived in the early hours of the morning, and also at nighttime to a slightly lesser extent.29 Accordingly, symptom distribution during the day has been identified as a potential PRO in COPD. In particular, morning and nighttime symptoms have emerged as potential new therapeutic targets. The problem with these new PROs is that instruments for evaluating them have only recently been developed; therefore, many clinical trials have used non-validated scales, as discussed below.
Efficacy of aclidinium bromide
Aclidinium (LAS34273; 3R-(2-hydroxy-2,2-di-thiophen-2-yl-acetoxy)-1-(3-phenoxy-propyl)-1-azonia-bicyclo [2.2.2] octane bromide) is an LAMA developed by a Spanish company (Almirall, Barcelona, Spain) available in a multidose dry powder device (Genuair®) administrated at a dose of 400 μg every 12 hours.
Preclinical studies
Preclinical studies confer characteristics such as a high affinity for muscarinic M3 receptors that make it well suited for use as a maintenance bronchodilator in COPD. In addition, it is longer lasting than ipratropium, but not as long as tiotropium, and onset of its biological effect is as fast as that of ipratropium.30 One main feature of aclidinium is its rapid hydrolysis in plasma. Aclidinium has shown to be rapidly hydrolyzed in human plasma to give two products, a carboxylic acid (LAS34850) and an alcohol (LAS34823), with an average disappearance time of 2.4 minutes in culture from human plasma. These two derivatives have no effect on muscarinic receptors,31 and therefore are not attributed any role in systemic effects. In clinical pharmacokinetic studies in patients with COPD, plasma concentrations decline rapidly with a half-life of between 1 hour and 3 hours.32 This suggests that aclidinium may have a reduced systemic exposure and could therefore be less susceptible to adverse effects.
Clinical studies vs placebo
The efficacy of aclidinium vs placebo was studied in three Phase III clinical trials, the AClidinium in Chronic Obstructive Respiratory Disease (ACCORD) I and II (studying aclidinium vs placebo over 12 weeks to assess the effect on trough FEV1 as the primary endopoint), and the Aclidinium To Treat Airway obstruction In COPD patieNts (ATTAIN) study, studying aclidinium vs placebo over 24 weeks to assess the effect on trough FEV1 as the primary endpoint). The results of all three studies are consistent, showing improvement in lung function and other clinical outcomes as summarized in Table 3. As an example, the ATTAIN study showed an average increase of 128 mL from baseline after 24 weeks of treatment with aclidinium, improving TDI by one point (Figure 2) and the St George’s Respiratory Questionnaire (SGRQ) by 4.6 units35: all these comparisons were clinically significant (see below). Regarding the impact on exacerbations, the ATTAIN study showed a 33% reduction in the total number of exacerbations. Very recently, this reduction in exacerbations has been reported for both reported and unreported exacerbations.36 However, a recent Cochrane systematic review indicates that this impact on exacerbations is mainly on hospital admissions.37 Additionally, the use of rescue medication fell in the aclidinium group, with an increase of 11% of rescue medication-free days in the active treatment group (see below). Finally, the ACCORD COPD I study showed a reduction in both nocturnal and early morning symptoms and respiratory symptoms throughout the day compared to placebo.33 These lung function, health status, and use of rescue medication findings were later confirmed in two further 12-month studies.38,39
  | Figure 2 Change from baseline in the TDI focal score over 24 weeks. |
Comparative studies vs tiotropium
As of completion of this review, three comparative studies have been published evaluating aclidinium Genuair vs tiotropium Handihaler. The first was a small Phase IIa study, in which 30 patients were randomized to compare both LAMAs in lung function.40 The second was a Phase IIIb study, in which 414 patients were randomized for 6 weeks in order to compare lung function in both treatments.41 The results of these studies indicate that both LAMAs are good bronchodilators, with no significant differences in bronchodilation achieved within 24 hours, as assessed by the area under the FEV1 curve between 0 hour and 24 hours. Although tiotropium showed greater functional gain in the first 12 hours after inhalation, aclidinium had better lung function in the second 12 hours, but without reaching statistical significance at 6 weeks of treatment, and with no clear relevant impact on symptoms (except for a decrease in cough and sputum for aclidinium) and use of rescue medication between the two molecules.41 It follows that both bronchodilators tiotropium and aclidinium have a similar efficacy profile over 6 weeks of treatment.
The third study, tiotropium vs aclidinium study, evaluated the rapidity of onset of action of both bronchodilators. Although in preclinical studies aclidinium was shown to be as rapid as ipratropium, in patients with COPD, both aclidinium and tiotropium bronchodilators showed a similar effect, with benefits in lung function and symptoms observable 10 minutes after dosing.42
Safety and tolerability
From the point of view of safety, aclidinium has a good safety profile in both 12-week and 24-week studies33,35 and the 12-month extensions.38,39 The main adverse effects from the ATTAIN and ACCORD COPD I studies are summarized in Table 4. Anticholinergic side effects were rare. With aclidinium 400 μg every 12 hours, the cholinergic adverse effects were dry mouth (2.7%) and constipation (1.7%). The most commonly reported adverse effect was exacerbation of COPD, which was reduced in the aclidinium group. Cough was infrequent and did not differ from placebo. Aclidinium was not associated with any infectious adverse effects. Systemic adverse effects were also low in frequency except for headache, which increased in the aclidinium arm of the ATTAIN study.
PROs with aclidinium
As a result of the above-mentioned efficacy results, improvements in PROs can also be inferred from the pivotal aclidinium studies. Information on dyspnea, rescue medication use, nighttime symptoms, and quality of life can be extracted from these trials and are summarized in Table 3.
Dyspnea is evaluated by TDI score24 in the aclidinium trials. During the 24-week ATTAIN trial,35 aclidinium gave greater significant improvement from baseline in TDI score compared to placebo (Figure 2). The improvement over placebo in baseline-adjusted mean (standard deviation) TDI score at week 24 was 1.0 (0.3) unit for aclidinium 400 µg. Additionally, more patients treated with aclidinium 400 µg had a clinically significant improvement of ≥1 unit in TDI at the end of the trial compared to placebo (56.9% vs 45.5%). A similar result was obtained in the ACCORD COPD I study,33 in which aclidinium significantly improved TDI scores compared to placebo at the majority of visits, and a higher percentage of patients in the aclidinium group (48%) achieved a clinically meaningful improvement in TDI of ≥1 unit compared to placebo.
The reduction in rescue medication was also evaluated. In the ATTAIN study,35 the mean total daily use of relief medication was significantly reduced by 0.95 puffs/day from baseline frequency with aclidinium 400 mg over 24 weeks (P<0.0001) compared to placebo. Accordingly, the percentage of days without the need for relief medication (11%) over 24 weeks was significantly increased vs placebo. Similarly, in the ACCORD COPD I study, aclidinium 400 μg significantly reduced total daily rescue medication use by 0.9 puffs/day vs placebo over the 12-week period (P<0.0001). These results were confirmed in a 12-month extension study in the ACCORD COPD I cohort.38 In this extension trial, mean rescue medication use in subjects taking aclidinium was 2.2 puffs/day. Additionally, a further 12-month trial39 showed a decrease in medication use of approximately one-half of the baseline value during the treatment period. A small 6-week study including 414 patients has recently evaluated the impact of aclidinium and tiotropium on relief medication use with similarly significant improvements for both bronchodilators and placebo.41
Nighttime symptoms were assessed in the ACCORD COPD I study.33 Compared to placebo, aclidinium significantly reduced frequency of nighttime symptoms by the end of study, including breathlessness, cough, sputum production, and wheezing. This improvement was observed for both the frequency and the severity of these symptoms, with a reduced number of awakenings. A small 6-week study including 414 patients has recently evaluated the impact of aclidinium and tiotropium on nighttime and morning symptoms with similar significant improvements for both bronchodilators and placebo.41
Health-related quality of life was measured using the SGRQ. In the ATTAIN study,35 significantly greater improvements from baseline in mean total SGRQ scores were observed with aclidinium at all time points (Figure 3). By the end of the trial, the baseline-adjusted total SGRQ score for subjects taking aclidinium 400 µg (P<0.0001) had improved by 4.6 (1.1) units vs placebo. Accordingly, more patients had a clinically significant improvement in total SGRQ score of ≥4 units at week 24 with aclidinium 400 µg (57.3%) compared to placebo (41.0%). A similar finding was observed in the ACCORD COPD I study,33 in which total SGRQ score for aclidinium 400 μg improved by 2.5 units vs placebo (P=0.019) by study end. Again, a higher percentage of patients taking aclidinium achieved a clinically meaningful improvement in total SGRQ scores of ≥4 units from baseline compared to placebo. These results were confirmed in a 12-month extension study in the ACCORD COPD I cohort.38 Patients who received continuous treatment with aclidinium 400 μg showed clinically significant improvements from baseline in total SGRQ scores, with an improvement of 7.9 units observed by the end of the study. Additionally, a further 12-month trial39 showed clinically important improvements with aclidinium 400 µg in total SGRQ scores at all study visits throughout the 52-week treatment period. In this trial, the mean improvement from baseline in total SGRQ score was 5.2 units for aclidinium 400 µg by study end. As in previous studies, the percentage of patients who achieved a clinically important improvement in total SGRQ score was numerically higher with aclidinium 400 µg.
  | Figure 3 Change from baseline in the SGRQ total score over 24 weeks. |
As for the inhaler device, aclidinium presented for inhalation via a multidose dry powder device (Genuair) reached a high peak inspiratory flow,43 ensuring good lung deposition even in more peripheral areas of the lung44 with a high degree of patient acceptance.45
Finally, adherence to aclidinium is another aspect to focus on. Unfortunately, few studies have been conducted on treatment adherence with different inhaled medications. Adherence studies with salmeterol/fluticasone and tiotropium46 and tiotropium alone have been conducted.47 Additionally, other recent adherence studies have included long-acting bronchodilators.48 More importantly, several studies have evaluated the degree of adherence to inhaled medication and associated factors. All these studies concur on three points: first, adherence to inhaled medication is poor;49 second, poor adherence has considerable impact on both patients46,50 and the health system;51 and third, adherence is a complex concept, in which the efficacy of the drug is important,52 but is not the only factor. Other factors influencing treatment adherence include patient satisfaction with the inhaler,53 the number of inhalers used,54 less-frequent dosing schedules,55 informal caregivers support,56 patient perception of clinician expertise,57 and certain comorbidities,58 among others.59 In this scenario, there is initially no reason to think that aclidinium adherence will differ from that of other twice-daily medications available for COPD, although studies are needed to show this. However, adherence is a much more complex concept than mere dose regimen or inhaler technique, and future trials should provide information on treatment adherence with this drug.
Conclusion
Aclidinium is a novel LAMA for the treatment of COPD, which has proven efficacy in objective functional measurements and PRO and a good safety profile. Comparison studies with tiotropium have shown it to have similar lung function improvement and a similar impact on PRO, including quality of life or symptom perception. Several new bronchodilators for the treatment of COPD are now available, and although the number of molecules and inhaler devices may grow considerably as a result, this will be of benefit to both patients and doctors, and give clinicians more tools to adapt therapy to the patients’ needs, and not vice versa.
Disclosure
JLLC has received honoraria for lecturing, scientific advice, participation in clinical studies, or writing for publications of (in alphabetical order): Almirall, AstraZeneca, Bayer, Boehringer Ingelheim, Cantabria Pharma, Chiesi, Esteve, Faes, Ferrer, GlaxoSmithKline, Menarini, MSD, Novartis, Pfizer, and Takeda. The rest of the authors declare no conflicts of interest.
References
Lopez-Campos JL, Ruiz-Ramos M, Soriano JB. Mortality trends in chronic obstructive pulmonary disease in Europe, 1994–2010: a joinpoint regression analysis. Lancet. 2014;2(1):54–62. | ||
Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. | ||
Janson C, Marks G, Buist S, et al. The impact of COPD on health status: findings from the BOLD study. Eur Res J. 2013;42(6):1472–1483. | ||
Lopez-Campos JL, Hartl S, Pozo-Rodriguez F, Roberts CM; European COPD Audit team. Variability of hospital resources for acute care of COPD patients: the European COPD audit. Eur Res J. 2014;43(3):754–762. | ||
Pauwels RA, Löfdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society study on chronic obstructive pulmonary disease. N Engl J Med. 1999;340(25):1948–1953. | ||
Rabe KF, Hurd S, Anzueto A, et al; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6): 532–555. | ||
Vestbo J, Anderson W, Coxson HO, et al; ECLIPSE investigators. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Res J. 2008;31(4):869–873. | ||
Agusti A, Calverley PM, Celli B, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. | ||
Lopez-Campos JL, Bustamante V, Munoz X, Barreiro E. Moving towards patient-centered medicine for COPD management: multidimensional approaches versus phenotype-based medicine-a critical view. Chronic Obstr Pulm Dis. 2014;11(5):591–602. | ||
Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Zogg S, Durr S, Miedinger D, Steveling EH, Maier S, Leuppi JD. Differences in classification of COPD patients into risk groups A–D: a cross-sectional study. BMC Res Notes. 2014;7(1):562. | ||
Jones P, Miravitlles M, van der Molen T, Kulich K. Beyond FEV(1) in COPD: a review of patient-reported outcomes and their measurement. Int J Chron Obstruct Pulmon Dis. 2012;7:697–709. | ||
Singer JP, Yusen RD. Defining patient-reported outcomes in chronic obstructive pulmonary disease: the patient-centered experience. Med Clin North Am. 2012;96(4):767–787. | ||
Rutten-van Molken MP, Oostenbrink JB, Miravitlles M, Monz BU. Modelling the 5-year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. Eur J Health Econ. 2007;8(2):123–135. | ||
Alagha K, Palot A, Sofalvi T, et al. Long-acting muscarinic receptor antagonists for the treatment of chronic airway diseases. Ther Adv Chronic Dis. 2014;5(2):85–98. | ||
Miravitlles M, Soler-Cataluna JJ, Calle M, et al. Spanish guideline for COPD (GesEPOC). Update 2014. Arch Bronconeumol. 2014;50(suppl 1):1–16. | ||
Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. | ||
Bedard ME, Brouillard C, Pepin V, et al. Tiotropium improves walking endurance in COPD. Eur Respir J. 2012;39(2):265–271. | ||
Mathioudakis AG, Kanavidis P, Chatzimavridou-Grigoriadou V, et al. Tiotropium HandiHaler improves the survival of patients with COPD: a systematic review and meta-analysis. J Aerosol Med Pulm Drug Deliv. 2014;27(1):43–50. | ||
Wise RA, Anzueto A, Cotton D, et al; TIOSPIR Investigators. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369(16):1491–1501. | ||
Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48(9):331–337. | ||
Hardin M, Cho M, McDonald ML, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44(2):341–350. | ||
Fletcher CM. The clinical diagnosis of pulmonary emphysema; an experimental study. Proc R Soc Med. 1952;45(9):577–584. | ||
Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751–758. | ||
Witek TJ Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J. 2003;21(2):267–272. | ||
Dobbels F, de Jong C, Drost E, et al; PROactive consortium. The PROactive innovative conceptual framework on physical activity. Eur Respiratory journal. 2014;44(5):1223–1233. | ||
Crawford B, Monz B, Hohlfeld J, et al. Development and validation of a cough and sputum assessment questionnaire. Eur Respir Med. 2008;102(11):1545–1555. | ||
Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264–272. | ||
Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin. 2009;25(8):2043–2048. | ||
Gavaldà A, Miralpeix M, Ramos I, et al. Characterization of aclidinium bromide, a novel inhaled muscarinic antagonist, with long duration of action and a favorable pharmacological profile. J Pharmacol Exp Ther. 2009;331(2):740–751. | ||
Sentellas S, Ramos I, Albertí J, et al. Aclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: in vitro plasma inactivation and pharmacological activity of its main metabolites. Eur J Pharm Sci. 2010;39(5):283–290. | ||
de la Motte S, Beier J, Schmid K, Pascual S, Jansat JM, Gil EG. Pharmacokinetics and safety of aclidinium bromide in younger and elderly patients with chronic obstructive pulmonary disease. Int J Clin Pharmacol Ther. 2012;50(6):403–412. | ||
Kerwin EM, D’Urzo AD, Gelb AF, et al; ACCORD I study investigators. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). Chronic Obstr Pulm Dis. 2012;9(2):90–101. | ||
Rennard SI, Scanlon PD, Ferguson GT, et al. ACCORD COPD II: a randomized clinical trial to evaluate the 12-week efficacy and safety of twice-daily aclidinium bromide in chronic obstructive pulmonary disease patients. Clin Drug Investig. 2013;33(12):893–904. | ||
Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40(4):830–836. | ||
Jones PW, Lamarca R, Chuecos F, et al. Characterisation and impact of reported and unreported exacerbations: results from ATTAIN. Eur Respir J. 2014;44(5):1156–1165. | ||
Ni H, Soe Z, Moe S. Aclidinium bromide for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;9:CD010509. | ||
D’Urzo A, Kerwin E, Rennard S, He T, Gil EG, Caracta C. One-year extension study of ACCORD COPD I: safety and efficacy of two doses of twice-daily aclidinium bromide in patients with COPD. Chronic Obstr Pulm Dis. 2013;10(4):500–510. | ||
Gelb AF, Tashkin DP, Make BJ, et al; LAS-MD-35 Study Investigators. Long-term safety and efficacy of twice-daily aclidinium bromide in patients with COPD. Respir Med. 2013;107(12):1957–1965. | ||
Fuhr R, Magnussen H, Sarem K, et al. Efficacy of aclidinium bromide 400 ug twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest. 2012;141(3):745–752. | ||
Beier J, Kirsten AM, Mróz R, et al. Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled Phase IIIb study. Chronic Obstr Pulm Dis. 2013;10(4):511–522. | ||
Vestbo J, Vogelmeier C, Creemers J, Falques M, Ribera A, Gil EG. Onset of effect of aclidinium, a novel, long-acting muscarinic antagonist, in patients with COPD. Chronic Obstr Pulm Dis. 2010;7(5):331–336. | ||
Magnussen H, Watz H, Zimmermann I, et al. Peak inspiratory flow through the Genuair inhaler in patients with moderate or severe COPD. Respir Med. 2009;103(12):1832–1837. | ||
Newman SP, Sutton DJ, Segarra R, Lamarca R, de Miquel G. Lung deposition of aclidinium bromide from Genuair, a multidose dry powder inhaler. Respiration. 2009;78(3):322–328. | ||
van der Palen J, Ginko T, Kroker A, et al. Preference, satisfaction and errors with two dry powder inhalers in patients with COPD. Expert Opin Drug Deliv. 2013;10(8):1023–1031. | ||
Ismaila A, Corriveau D, Vaillancourt J, et al. Impact of adherence to treatment with tiotropium and fluticasone propionate/salmeterol in chronic obstructive pulmonary diseases patients. Curr Med Res Opin. 2014;30(7):1427–1436. | ||
Laforest L, Licaj I, Devouassoux G, Hartwig S, Marvalin S, Van Ganse E. Factors associated with early adherence to tiotropium in chronic obstructive pulmonary disease. Chron Respir Dis. 2013;10(1):11–18. | ||
Wurst KE, St Laurent S, Mullerova H, Davis KJ. Characteristics of patients with COPD newly prescribed a long-acting bronchodilator: a retrospective cohort study. Int J Chron Obstruct Pulmon Dis. 2014;9:1021–1031. | ||
de Miguel-Díez J, Jiménez-García R, Hernández-Barrera V, et al. Clustering of unhealthy lifestyle behaviors is associated with a low adherence to recommended preventive practices among COPD patients in Spain. Chronic Obstr Pulm Dis. 2014;11(4):459–467. | ||
Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–943. | ||
van Boven JF, Tommelein E, Boussery K, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Respir Res. 2014;15:66. | ||
Huetsch JC, Uman JE, Udris EM, Au DH. Predictors of adherence to inhaled medications among veterans with COPD. J Gen Intern Med. 2012;27(11):1506–1512. | ||
Chrystyn H, Small M, Milligan G, Higgins V, Gil EG, Estruch J. Impact of patients’ satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med. 2014;108(2):358–365. | ||
Yu AP, Guérin A, Ponce de Leon D, et al. Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long-acting maintenance inhalers. J Med Econ. 2011;14(4):486–496. | ||
Agh T, Inotai A, Meszaros A. Factors associated with medication adherence in patients with chronic obstructive pulmonary disease. Respiration. 2011;82(4):328–334. | ||
Trivedi RB, Bryson CL, Udris E, Au DH. The influence of informal caregivers on adherence in COPD patients. Ann Behav Med. 2012;44(1):66–72. | ||
Cecere LM, Slatore CG, Uman JE, et al. Adherence to long-acting inhaled therapies among patients with chronic obstructive pulmonary disease (COPD). Chronic Obstr Pulm Dis. 2012;9(3):251–258. | ||
Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur J Clin Pharmacol. 2012;68(10):1365–1373. | ||
Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. | ||
Borg GA. Perceived exertion: a note on “history” and methods. Med Sci Sports. 1973;5(2):90–93. | ||
Gift AG. Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs. 1989;14(6):323–325. | ||
Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42(10):773–778. | ||
Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med. 1991;85(suppl B):25–31; discussion 33–37. | ||
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. | ||
van der Molen T, Willemse BW, Schokker S, ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the clinical COPD questionnaire. Health Qual Life Outcomes. 2003;1:13. | ||
Hernandez P, Balter M, Bourbeau J, Hodder R. Living with chronic obstructive pulmonary disease: a survey of patients’ knowledge and attitudes. Respir Med. 2009;103(7):1004–1012. | ||
Partridge MR, Miravitlles M, Stahl E, Karlsson N, Svensson K, Welte T. Development and validation of the capacity of daily living during the morning questionnaire and the global chest symptoms questionnaire in COPD. Eur Respir J. 2010;36(1):96–104. | ||
Pommer AM, Prins L, van Ranst D, et al. Development and validity of the patient-centred COPD questionnaire (PCQ). J Psychosom Res. 2013;75(6):563–571. | ||
Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339–343. | ||
Pokrzywinski RF, Meads DM, McKenna SP, Glendenning GA, Revicki DA. Development and psychometric assessment of the COPD and Asthma Sleep Impact Scale (CASIS). Health Qual Life Outcomes. 2009;7:98. | ||
Hareendran A, Palsgrove AC, Mocarski M, et al. The development of a patient-reported outcome measure for assessing nighttime symptoms of chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2013;11:104. | ||
Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(suppl 1):S79–S83. | ||
Al-shair K, Kolsum U, Berry P, et al. Development, dimensions, reliability and validity of the novel Manchester COPD fatigue scale. Thorax. 2009;64(11):950–955. | ||
Whitehead L. The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage. 2009;37(1):107–128. | ||
Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. | ||
Weldam SW, Schuurmans MJ, Liu R, Lammers JW. Evaluation of Quality of Life instruments for use in COPD care and research: a systematic review. Int J Nurs Stud. 2013;50(5):688–707. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.