Back to Journals » Nutrition and Dietary Supplements » Volume 7
Parenteral nutrition in intestinal failure
Authors Kurkchubasche A, Herron T, Winkler M
Received 24 September 2014
Accepted for publication 9 December 2014
Published 16 January 2015 Volume 2015:7 Pages 11—20
DOI https://doi.org/10.2147/NDS.S55098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gary Johanning
Arlet G Kurkchubasche,1 Thomas J Herron,2 Marion F Winkler3
1Department of Surgery and Pediatrics, 2Department of Surgery, Alpert Medical School of Brown University, 3Department of Surgery/Nutritional Support Service, Rhode Island Hospital, Providence, RI, USA
Abstract: Intestinal failure is a consequence of extensive surgical resection resulting in anatomic loss and/or functional impairment in motility or absorptive capacity. The condition is clinically characterized by the inability to maintain fluid, energy, protein, electrolyte, or micronutrient balance when on a conventionally accepted, normal diet. Parenteral nutrition (PN) is the cornerstone of management until intestinal adaptation returns the patient to a PN-independent state. Intestinal length, residual anatomic segments and motility determine the need for and duration of parenteral support. The goals of therapy are to provide sufficient nutrients to enable normal growth and development in children, and support a healthy functional status in adults. This review addresses indications for PN, the formulation of the PN solution, patient monitoring, and considerations for prevention of PN-associated complications. With the ultimate goal of achieving enteral autonomy, the important role of diet, pharmacologic interventions, and surgery is discussed.
Keywords: intestinal failure, short-bowel syndrome, parenteral nutrition, home nutrition support, intestinal rehabilitation
Introduction
Parenteral nutrition (PN) is life-sustaining therapy for patients who have intestinal failure (IF). This includes not only the traditional short-bowel syndrome (SBS) patients, who have IF on the basis of anatomic loss (congenital or acquired), but also those patients with functional impairment in motility or absorptive capacity.1,2 The condition is clinically characterized by the inability to maintain fluid, energy, protein, electrolyte, or micronutrient balance when on a conventionally accepted, normal diet.1 A functional definition of IF, which is also the basis for reimbursement, arbitrarily requires PN dependence.3
There are multiple etiologies of IF; however, for the majority of patients, the final common pathway is massive bowel resection. Consequently, the goal of IF management is to support the patient’s physiologic needs during this acute period of stress and to attempt to achieve enteral autonomy, if feasible. PN is the primary modality of support until intestinal absorptive function is restored. This process of intestinal adaptation is impacted by the residual intestinal length and motility of the intestine and the patient’s tolerance for and adherence to dietary and pharmacologic therapies.4 An understanding of normal physiology (Table 1) is fundamental to providing care to these complex patients.5 Surgical interventions to restore intestinal continuity and improve function are requisite components of any comprehensive intestinal rehabilitation program.3,6 This review provides the fundamental components of PN support and highlights the key differences in the management of IF in children and adults.
  | Table 1 Normal gastrointestinal physiology |
Etiology of intestinal failure
IF as the consequence of surgical resection most commonly occurs in adults after injury to the intestine (ischemic or traumatic), inflammatory bowel disease (IBD), malignancy, and radiation therapy. IF may also be a consequence of complications following bariatric surgery.7,8 Motility disorders, such as chronic intestinal pseudo-obstruction, constitute the remainder of cases. In children, congenital disorders that predispose to IF include malrotation with volvulus, gastroschisis, intestinal atresia, and long-segment Hirschsprung’s disease. Preterm infants are particularly vulnerable to IF as a consequence of necrotizing enterocolitis. Disorders of metabolism, motility, and cellular structure (tufting enteropathy) comprise another subset of pediatric patients with IF. Knowledge of the incidence of IF is hampered by the lack of reliable data, but this is considered to be a rare condition on the basis of estimates of PN-dependent individuals.9 The North American Home Parenteral and Enteral Nutrition registry reported 5,481 patients on home PN (HPN) between 1985 and 1992.10 Some of the leading conditions at the time included Crohn’s disease, malignancy, congenital disorders, dysmotility, acquired immunodeficiency syndrome, and intestinal ischemia. Attempts to delineate the extent of the current use of HPN in the US are under way via the Sustain Patient Care Registry.11 The majority (42%) of the 1,250 registry patients enrolled in the first 2.5 years received HPN because of SBS.
Indication for parenteral nutrition
Intestinal length and site of resection will impact absorption and motility, and influence an individual’s need for intravenous (IV) fluid and PN support.12,13 Greater absorption occurs with more remaining bowel, which is directly related to the potential for becoming PN-independent.14–16 The residual anatomic segment of the intestine impacts the potential for adaptation, and may preserve vital functions, such as vitamin B12 absorption. The presence of the ileocecal valve (ICV) suggests that much of the colon is present, adding valuable mucosal surface area for water and nutrient assimilation. It not only provides a brake for intestinal transit but also protects the ileum from the high microbial content of the colon.
The nutritional goals for patients with IF are to provide adequate fluid, macronutrients, and micronutrients and to prevent dehydration and nutrient deficiencies.17 In the pediatric patient, there is the additional requirement to support normal growth and development. Patients may present with premorbid malnutrition attributed to chronic disease (IBD, malignancy) or treatment (radiation enteritis), or they may be previously healthy individuals who have sustained an acute intestinal injury (mesenteric ischemia, trauma) or neonatal conditions. Energy and nutrient goals are determined by assessment of nutrition status, activity level, and ongoing metabolic demands for recovery, growth, and development. Individuals with IF may experience initial gastric hypersecretion and rapid gastric emptying, resulting in the need to compensate for substantial fluid, electrolyte, and micronutrient deficits.18,19 Osmotic diarrhea may also result from carbohydrate (CHO) malabsorption and colonic fermentation of unabsorbed CHO.20 Steatorrhea occurs due to unabsorbed bile salts and fat, and is accompanied by fat-soluble vitamin deficiencies and may also be complicated by excess oxalate absorption from the colon resulting in calcium oxalate kidney stones.21
Formulation of the parenteral nutrition solution
A decision-making algorithm to aid in the formulation of the PN prescription is shown in Figure 1. The initial amount of CHO given for an adult is usually 150–200 g/day, with increases made over 72–96 hours based on energy requirements and glycemic control.22 Neonates start with a CHO demand of 18–25 g/kg/day, and this is administered depending on the tolerated glucose-infusion rate, which ideally should not exceed 15 mg/kg/min.23 The initial parameters for protein provision are generally quoted as 0.8–1.5 g/kg/day for adults. Requirements for neonates, infants, and children are determined based on estimated gestational age, chronologic age, and underlying conditions, and range from 2–4 g protein/kg/day. Both adults and children are provided with 1 g/kg/day of lipid emulsion.3,23,24 This is a reduction from the more liberal amounts previously provided in an effort to avert hepatobiliary dysfunction. All patients require daily multivitamin, mineral, and trace-element supplementation via PN. The amount of IV fluid or PN volume is determined according to baseline fluid requirements and known gastrointestinal (GI) tract losses from stoma, stool, or other forms of GI decompression. The goal is to assure adequate urine output (>1,000 mL/day in adults, >1 mL/kg/hour in children) and urine sodium >20 mEq/L.23,25,26
  | Figure 1 Decision-making algorithm for establishing initial parenteral nutrition formulation. |
PN formulations can be compounded as a three-in-one total nutrient admixture, containing amino acids, dextrose, and lipid, or as a two-in-one solution with only amino acids and dextrose.27 Both types of formulations may be used in the immediate postoperative period, but a total nutrient admixture is more convenient for the stable home patient. The lipid emulsion is provided separately in this instance. The international commercial availability of IV fat emulsion (IVFE) differs. In the US, standard IVFE products are primarily made from soybean oil that contains high concentrations of polyunsaturated fatty acids, omega-6 fatty acids, and phytosterols.24,28 Alternative solutions are widely available in other parts of the world, in which the soybean oil has been partially replaced by medium-chain triglycerides, olive oil, and fish oil.29 The recommended dosage of IVFE varies; however, current guidelines suggest limiting the lipid dose to <1 g/kg/day for adults and for children to minimize hepatic complications.3,25 This lower lipid dose is adequate to meet essential fatty-acid requirements.
The electrolyte composition of PN mixtures for IF patients typically requires individualization, because of the variation in intestinal absorption and magnitude of diarrheal losses.27,30 In the postoperative period, fluid, electrolytes, and acid–base balance should be corrected before initiating PN. Repletion doses of magnesium, phosphorus, and potassium may be required in the first few days. Only patients who no longer require frequent changes in the PN formulation are considered stable for discharge. Even for the stable patient at home, electrolytes may be periodically adjusted to correct electrolyte imbalances or acid–base disorders resulting from diarrhea. Table 2 illustrates daily electrolyte and mineral requirements for pediatric and adult patients, assuming normal age-related organ function.
  | Table 2 Daily parenteral nutrition electrolyte and mineral requirements for pediatric and adult patients |
PN is typically infused over a 24-hour period in the postoperative period. In the home setting, the rate of PN infusion for adults is cycled (usually overnight) between 8–16 hours, to allow greater freedom during the day for activities of daily living.25 Glycemic control and fluid tolerance are important considerations in determining the length of infusion. An added benefit from cycled PN is the prevention of hepatobiliary complications by promoting mobilization of fat and fatty-acid oxidation during the time PN is not infusing.31 Infants need sufficient glycogen stores or need to be on a baseline enteral infusion to safely tolerate PN cycling and avoid hypoglycemia, which may otherwise result in devastating seizures.
Preparing for home parenteral nutrition
HPN is necessary for patients who are unable to maintain hydration, nutritional status, or weight without IV fluids and nutrients. It serves to correct preexisting malnutrition and facilitate ongoing recovery and growth.24,32–34 A thorough assessment of the home environment, the patient’s support systems, and insurance coverage is necessary before HPN discharge.25,35 Optimizing the organization and safety of the home environment decreases HPN therapy-related anxiety, facilitates learning, and promotes independence.35 Administering PN at home requires rigorous, daily care with stringent aseptic technique, because of the indwelling central venous catheter and risks of infection.
Patients and caregivers should be provided with information about how to contact the home-care company, home-infusion nurses, reasons to call for help, how to decrease risk, and how to promote adherence and compliance with nutrition support-related procedures.35 Independence with HPN therapy is achieved through comprehensive patient and family education addressing care of the access device and site, troubleshooting equipment, learning to reliably monitor weight, hydration status, blood, or urine glucose, recognizing early signs of infection, encouraging a diet to promote intestinal adaptation, and engaging in physical activity.35
Long term HPN requires coordinated monitoring and communication among the patient, family, home-infusion nurses and pharmacists, and the physician and nutrition-support team.36 Routine home-nursing visits and physician or clinic follow-up are essential for prevention, early detection, and treatment of medical complications and identification of psychosocial stressors. Depression and impaired quality of life may occur because of chronic illness and dependence on complex technology required for HPN.37 Ongoing communications with the patient and their caregivers are important to help them adapt and adequately cope with these lifestyle adjustments. Patient-support organizations and online resources are widely available.
Patient monitoring
A multifaceted patient assessment includes evaluation of their hydration status and interval growth and development. This requires the serial documentation of weight and height. Performance status is assessed by obtaining a patient history, with a review of systems focusing on activities of daily living and age-specific developmental milestones. Nutritional assessment should include evaluation of food diaries and documentation of all enteral and parenteral sources of intake.38,39 Daily losses from stomal diversion, fecal losses, and urinary outputs must be recorded and compensated for. The focus of laboratory studies is to facilitate provision of the correct fluid and electrolyte composition. These studies also serve to assess nutrition status and organ function. Laboratory monitoring is conducted at weekly intervals in the month following discharge, but most stable HPN patients can eventually be managed with less frequent blood draws (monthly or quarterly), unless the clinical condition dictates otherwise (Table 3).40 Seasonal variation in temperature and acute illness may require more aggressive monitoring of fluid and electrolytes.
Routine monitoring of trace elements and vitamins has been limited to situations in which there is clinical suspicion of deficiency or toxicity. Clinicians should be aware of the potential for metabolic bone disease and the risk of vitamin D deficiency and osteoporosis among IF patients.41–45 These risks necessitate periodic monitoring of serum levels of 25-hydroxyvitamin D and bone densitometry (for adults). The recent drug shortages and rationing of parenteral trace-element and vitamin components create a need for more vigilant micronutrient monitoring.
Complications associated with parenteral nutrition
There are several categories of complications encountered in PN-supported patients. Derangements in fluid and electrolyte status are generally directly attributable to the PN formulation, and are heavily dependent on the patient’s physiologic intestinal function. Acid–base derangements are often attributed to the PN prescription; however, conditions such as D-lactic acidosis require an understanding of the patient’s intestinal microflora.46,47 Complications related to micronutrient, vitamin, and trace-element deficiencies might manifest as dermatologic conditions. The most concerning are those that potentially impact the central nervous system, such as manganese toxicity.23
Infection and hepatic impairment are multifactorial, but PN plays a significant role in both of these categories. Infection, specifically central line-associated bloodstream infections (CLABSI), can be either exogenous or endogenous. Exogenous sources relate to the care of the catheter, both at the skin-entry site and at the hub connections with the infusion line and infusate. Very specific guidelines exist for this component of patient management to minimize these risks.40,48,49 Endogenous sources of infection are derived from the patient’s own body, whether this be an abscess seeding the bloodstream or bacterial overgrowth from dysfunctional intestine that allows for bacterial transit into the circulation. In this case the catheter is the “innocent bystander”, upon which the organisms settle and colonize. Systemic interventions, such as management of indolent infections, drainage of abscesses, control of the microflora, and use of antimicrobial agents, such as antibiotic locks and ethanol locks, are considered essential.50,51 The patient’s underlying immune status naturally contributes to the efficacy of treatment, but can also be adversely impacted by an excess provision of lipids, which impair white blood-cell function.
Hepatic dysfunction is a feared PN complication, and used to be primarily attributed to the formulation; however, contemporary thinking acknowledges that this complication arises from the triad of the patient’s underlying disease process, repeated bouts of infection/sepsis, and the potentially hepatotoxic effects from the formulation.29 The patient’s disease process includes the natural sequelae of extensive bowel resection (cholestasis and development of gallstones) or intestinal obstruction, the effects of chemotherapy and radiation therapy, and the multiorgan involvement in some of the metabolic disorders, as well as in the chronic inflammatory diseases (IBD). Repeated episodes of CLABSI or other infections requiring repeated operative and nonoperative interventions subject the liver and other organs to stresses from the microbe as well as the medications employed to treat the infection.52 When sepsis develops, this in and of itself predisposes the patient to multisystem organ failure. The PN components and mode of administration have been the long-term focus in avoiding hepatic impairment.53,54 Research is now providing new knowledge of the immunomodulating effects of various lipid preparations. Limiting exposure to omega-6 lipids by either total lipid restriction, minimal lipid dosage, or by providing an alternate source of lipid with a more favorable immune profile containing omega-3 fatty acids and perhaps other non-soy, non-fish oil-based constituents may be hepatoprotective and even restore function.3,24,29 Hepatic dysfunction is most prevalent in the pediatric population, and places a constant end point to the horizon for restoring autologous intestinal function. If the threat of hepatic dysfunction can be delayed, this provides promise for some of these patients to successfully wean from their PN dependence.16
Management of oral diet
An aggressive attempt to wean PN and promote enteral autonomy should be undertaken in all patients.22,55–57 Some patients will be able to achieve nutritional adequacy with oral or enteral feeding, while others may be partially or totally PN-dependent. Encouragement of an oral diet is essential to promote intestinal adaptation; a condition in which the intestine undergoes hypertrophy and nutrient absorption is improved. Patients should eat small, frequent meals, minimize refined CHO and sugar, maximize complex CHO (soluble fiber), and sip fluids between meals.55,58 The macronutrient content of the diet, as well as fluid choice, is modified based on the presence or absence of a colon.58 Ideally, patients require isotonic, high-sodium fluids to maximize the potential for water and sodium absorption.59 Oral rehydration solutions that are used to treat diarrhea (commercially available or homemade) are preferred. These solutions typically contain 90–120 mM of sodium and 56 mM of glucose per liter.60 Patients should be instructed not to consume sport drinks that are too high in glucose and too low in sodium, and other hypertonic fructose-containing fluids or hypotonic juices and water, which draw sodium and water into the jejunum and increase fluid loss.
Eating is important even for those patients who remain dependent on PN as their primary form of nourishment.61 Clinicians, especially dietitians, should address the social and emotional context of food and mealtimes, as well as dietary adequacy, need for dietary modification or restriction, and control of GI symptoms. Quality of life may be enhanced by learning strategies for eating small amounts of food for comfort and taste, dining in restaurants, and avoiding social isolation due to the discomfort of not being able to eat.61 Children should be encouraged to sit at the table to watch others eat, help shop and prepare food, and play with food and utensils in order to encourage oral motor-skill development and familiarity with food and mealtimes.
Diet and enteral feeding
The focus in pediatric IF management is on the neonate and infant, as catastrophic loss of the intestine is much less frequent in the older child and adolescent. IF in this cohort is generally anatomic, and is therefore referred to as SBS. It occurs either on the basis of congenital absence or perinatal loss of bowel. Efforts to guide an infant to PN independence start early with provision of enteral substrate via gastrostomy tubes.3,32,34 Many infants with necrotizing enterocolitis are simply too premature to expect for them to be able to feed by mouth, and enteral access allows the clinician to specifically control the amount and composition of formula, many of which are not palatable to the child. The use of nasoenteric tubes is avoided when long-term support is anticipated.
Once the GI tract is amenable to enteral stimulation, low-volume feeds are initiated, optimally using breast milk when available. Gestationally appropriate formulas are employed, unless the intestine is not expected to handle complex CHO and proteins on the basis of mucosal damage or extreme SBS. While it is not clear specifically how complex nutrients contribute to the process of adaptation, provision of elemental nutrients may lead to a more rapid ability to absorb these nutrients and offset the prolonged PN use.25 Subsequent conversion to more complex nutrient intakes can then be initiated. The mode of feeding has also been a subject of controversy, with intermittent bolus feedings being considered most physiologic and perhaps most stimulating to the hormonal axis, which also controls hepatic and pancreatic secretions. However, the dysfunctional bowel of these infants may not have normal gastric emptying, which then contributes to feeding intolerance by exacerbating physiologic reflux, which is present in all infants. Continuous enteral feeds may obviate these obstacles and allow for a more continuous exposure of the intestine to nutrients.62 Combination regimens may include daytime bolus feeds with nighttime continuous feedings, or oral feeds in addition to continuous gastrostomy-tube feeds.23 Oral feedings (by bottle or breast), whether they be sham feeds (drained via gastrostomy tube) or valid feeds, play an incredible role in the bonding of parent and child.
As the infant progresses with its ability to tolerate the enteral load, the nutrients provided from PN can be reduced. This presents an opportunity to administer PN over a shorter time interval as cyclic PN, which is the first step toward preparing the infant for home. Cycling is important regardless of the tolerance of enteral feedings, but must be instituted with caution in the neonate who does not initially have the glycogen reserves to maintain blood glucose and prevent hypoglycemic seizure activity, which is the most detrimental to the developing brain. Close monitoring of growth and development is the mainstay of pediatric nutrition management, and adequate energy provision from all sources must be assured. At times, it is difficult to determine what the actual absorption of enteral nutrients is, and although it is important to push the envelope with feedings, the safety net of PN should not be withdrawn prematurely.
Pharmacological management
Medication management is frequently necessary to control GI symptoms and maximize absorption.20,55,63 The provision of fiber supplementation is an important modulator of stool consistency and volume. Fiber also provides a substrate for the production of short-chain fatty acids in the colon, particularly butyrate, which is known to enhance the function of colonocytes.4 Soluble fiber also provides a substrate for energy and enhances water and electrolyte absorption.64
Antacids (hydrogen antagonists or proton-pump inhibitors) are necessary to reduce gastric hypersecretion within the first 6 months following massive small-bowel resection. Antidiarrheal medications, taken 30–60 minutes prior to meals, are used to slow transit. Somatostatin (an antisecretory agent) may be effective in slowing intestinal transit and reducing diarrhea; however, this medication increases the risk of cholelithiasis and may interfere with intestinal adaptation.65 Pancreatic enzyme replacement may help digestion for those individuals who have steatorrhea resulting from rapid intestinal transit and reduced mixing of pancreatic enzymes with food. Bile-acid sequestrants may be used to manage diarrhea resulting from malabsorbed bile salts; however, these agents can actually worsen diarrhea in some patients and impair absorption of fat-soluble vitamins.66
Antibiotics or probiotics may be used for short-term treatment of bacterial overgrowth. Repeated cycles are often required; therefore, patients may be at risk of long-term side effects of antibiotics, such as changes in intestinal microflora, impaired immunity, and drug resistance.67
Newer pharmacological approaches, including the use of human growth factors and such hormones as glucagon-like peptide (GLP)-2, are intended to facilitate adaptation and have been shown to reduce PN dependency.68 GLP-2 (teduglutide) is a new targeted therapy that has been shown to increase intestinal villus height and crypt depth, thereby leading to enhanced absorptive capacity.69,70 The Food and Drug Administration in the US has recently approved the use of teduglutide for the treatment of PN-dependent adult patients with SBS who are clinically stable with nonobstructive and nonmalignant disease. The goal of this therapy is to reduce the volume and frequency of PN infusions in the setting of optimization of diet, hydration, and antidiarrheal medications.38
Surgical considerations in intestinal failure
There are literature-based estimates of minimal residual length of the intestine associated with achievement of enteral autonomy. This has been a moving target; however, it is generally accepted that the adult with 60–90 cm and the infant with 40 cm of small intestine and the ICV have the potential to become PN-independent.29 This depends not only on the length but also on the nature of the retained intestinal segment and whether it is in continuity with the colon. The presence of the ICV probably is a surrogate for the most adaptive segment, the terminal ileum being present, and suggests that the full length of the colon is present to participate in the management of fluid absorption. Since the length of the residual intestine correlates with PN dependence, surgeons make every effort to conserve length. Although enterostomy may be necessary, it should be reversed to optimize functional outcome and allow for colonic involvement in the absorptive process.
Secondary operations are intended to address the natural consequences of intestinal adaptation. These include progressive dilation of the intestine to increase the absorptive surface area and prolongation of the transit time. These features of intestinal adaptation become adverse consequences when stasis results in mucosal inflammation and bacterial overgrowth. This may interfere with tolerance of oral and enteral support and contribute to prolonged PN dependence. It also subjects the individual to an increased risk of infection and hepatic failure.
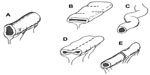
Numerous techniques have been described for autologous intestinal reconstruction.71 These techniques are based on the ability to manipulate the configuration of the intestine while preserving all luminal surface area. This can be accomplished by longitudinal enteroplasty creating two parallel conduits out of one dilated segment and reconfiguring them into a sequential configuration (Figure 2).72 This procedure risks loss of one or both segments as a consequence of ischemia. A more conservative but still controversial technique is the serial transverse enteroplasty procedure, which avoids manipulation of the blood supply and accordions the bowel into a thinner and longer conduit.73
Small-intestine transplantation with or without liver transplantation is considered when autologous reconstruction is not prudent or safe and the complications of sepsis, limited venous access, and liver dysfunction interfere with the ability to provide ongoing PN support.74 Maximal effort toward PN independence should precede referral for transplantation.
Conclusion
IF in children and adults remains a challenging problem, optimally addressed in a multidisciplinary manner.75,76 PN, while remaining the cornerstone of initial therapy, is associated with morbidity and mortality, and should be considered a bridge to enteral autonomy. Advancements made in the management of diet, pharmacologic interventions, and autologous surgery provide clinicians with many treatment options. All patients should benefit from targeted attempts at intestinal rehabilitation.
Disclosure
The authors report no conflicts of interest in this work.
References
O’Keefe SJ, Buchman AL, Fishbein TM, Jeejeebhoy KN, Jeppesen PB, Shaffer J. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol. 2006;4(1):6–10. | |
Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130(2 Suppl 1):S16–S28. | |
Modi BP, Jaksic T. Pediatric intestinal failure and vascular access. Surg Clin N Am. 2012;92(3):729–743. | |
Tappenden KA. Intestinal adaptation following resection. JPEN J Parenter Enteral Nutr. 2014;38(Suppl 1):23S–31S. | |
Tappenden KA. Pathophysiology of short bowel syndrome: considerations of resected and residual anatomy. JPEN J Parenter Enteral Nutr. 2014;38(Suppl 1):14S–22S. | |
Iyer KR. Surgical management of short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014;38(Suppl 1):53S–59S. | |
Raheem SA, Deen OJ, Corrigan ML, et al. Bariatric surgery complications leading to small bowel transplantation: a report of 4 cases. JPEN J Parenter Enteral Nutr. 2014;38(4):513–517. | |
Isom KA, Andromalos L, Ariagno M, et al. Nutrition and metabolic support recommendations for the bariatric patient. Nutr Clin Pract. 2014;29(6):718–739. | |
Jeppesen PB. Short bowel syndrome – characterization of an orphan condition with many phenotypes. Expert Opin Orphan Drugs. 2013;1(7):515–525. | |
Howard L, Ament M, Fleming CR, Shike M, Steiger E. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology. 1995;109(2):355–365. | |
Guenter P, Robinson L, DiMaria-Ghalili RA, Lyman B, Steiger E, Winkler MF. Development of SustainTM: ASPEN’s National Patient Registry for Nutrition Care. JPEN J Parenter Enteral Nutr. 2012;36(4):399–406. | |
Jeppesen PB. Spectrum of short bowel syndrome in adults: intestinal insufficiency to intestinal failure. JPEN J Parenter Enteral Nutr. 2014;38(Suppl 1):8S–13S. | |
Sondheimer JM, Cadnapaphornchai M, Sontag M, Zerbe GO. Predicting the duration of dependence on parenteral nutrition after neonatal intestinal resection. J Pediatr. 1998;132(1):80–84. | |
Carbonnel F, Cosnes J, Chevret S, et al. The role of anatomic factors in nutritional autonomy after extensive small bowel resection. JPEN J Parenter Enteral Nutr. 1996;20(4):275–280. | |
Messing B, Crenn P, Beau P, Boutron-Rualt MC, Rambaud JC, Matuchansky C. Long term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117(5):1043–1050. | |
Fallon EM, Mitchell PD, Nehra D, et al. Neonates with short bowel syndrome: an optimistic future for parenteral nutrition independence. JAMA Surg. 2014;149(7):663–670. | |
Sundaram A, Koutkia P, Apovian CM. Nutritional management of short bowel syndrome in adults. J Clin Gastroenterol. 2002;34(3):207–220. | |
Windsor CW, Fejfar J, Woodward DA. Gastric secretion after massive bowel resection. Gut. 1969;10(10):779–786. | |
Buxton B. Small bowel resection and gastric hypersecretion. Gut. 1974;15(3):229–238. | |
Jeejeebhoy KM. Short bowel syndrome: a nutritional and medical approach. CMAJ. 2002;166(10):1297–1302. | |
Nightingale JM. Management of patients with a short bowel. World J Gastroenterol. 2001;7(6):741–751. | |
Matarese LE. Nutrition and fluid optimization for patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2013;37(2):161–170. | |
Squires RH. Intestinal failure. In: Corkins MR, editor. The ASPEN Pediatric Nutrition Support Core Curriculum. Silver Spring (MD):American Society for Parenteral and Enteral Nutrition; 2010: 311–322. | |
Gabe SM. Lipids and liver dysfunction in patients receiving parenteral nutrition. Curr Opin Clin Nutr Metab Care. 2013;16(2):150–155. | |
Rhoda KM, Suryadevara S, Steiger E. Home parenteral nutrition support for intestinal failure. Surg Clin N Am. 2011;91(4):913–932. | |
Schwarz K, Ternberg J, Bell M, Keating J. Sodium needs of infants and children with ileostomy. J Pediatr. 1983;102(3):509–513. | |
Dibb M, Teubner A, Theis V, Shaffer J, Lal S. Review article: The management of long-term parenteral nutrition. Aliment Pharmacol Ther. 2013;37(6):587–603. | |
Clayton P, Whitfield P, Iyer K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition. 1998;14(1):158–164. | |
Thompson JS, Rochling FA, Weseman RA, Mercer DR. Current management of short bowel syndrome. Curr Probl Surg. 2012;49(2):52–115. | |
Scanzano C, Iacone R, Alfonsi L, et al. Composition of personalized and standard nutritional mixtures in patients on home parenteral nutrition. Eur J Clin Nutr. 2014;68(4):433–436. | |
Stout S, Cober M. Metabolic effects of cyclic parenteral nutrition infusion in adults and children. Nutr Clin Pract. 2010;25(3):277–281. | |
Goulet OJ, Revillon Y, Jan D, et al. Neonatal short bowel syndrome. J Pediatr. 1991;119(1):18–23. | |
Goulet O, Gobet B, Talbotec C, et al. Outcome and long-term growth after extensive small bowel resection in the neonatal period: a survey of 87 children. Eur J Pediatr Surg. 2005;15(2):95–101. | |
Spencer AU, Neaga A, Wet B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242(3):403–409. | |
Winkler M, Hagan E, Albina J. Home nutrition support. In: Mueller CM, editor. The ASPEN Adult Nutrition Support Core Curriculum. 2nd ed. Silver Spring (MD):American Society for Parenteral and Enteral Nutrition, 2012:639–655. | |
Winkler M, Guenter P. Long-term home parenteral nutrition: it takes an interdisciplinary approach. J Infus Nurs. 2014;37(5):389–395. | |
Winkler MF, Ross VM, Piamjariyakul U, Gajewski B, Smith CE. Technology dependence in home care: impact on patients and their family caregivers. Nutr Clin Pract. 2006;21(6):544–556. | |
Seidner DL, Schwartz LK, Winkler MF, Jeejeebhoy K, Boullata JI, Tappenden KA. Increased intestinal absorption in the era of teduglutide and its impact on management strategies in patients with short bowel syndrome-associated intestinal failure. JPEN J Parenter Enteral Nutr. 2013;37(2):201–211. | |
Parrish CR, DiBaise JK. Nutrition therapy for short bowel syndrome in the adult patient. Pract Gastroenterol. 2014;38(10):40–51. | |
Durfee SM, Adams SC, Arthur E, et al. ASPEN Standards for Nutrition Support: home and alternate site care. Nutr Clin Pract. 2014;29(4):542–555. | |
Ferrone M, Geraci M. A review of the relationship between parenteral nutrition and metabolic bone disease. Nutr Clin Pract. 2007;22(3):329–339. | |
Ament ME. Bone mineral content in patients with short bowel syndrome: the impact of parenteral nutrition. J Pediatr. 1998;132(3):386–388. | |
Ellegård L, Kurlberg G, Bosaeus I. High prevalence of vitamin D deficiency and osteoporosis in out-patients with intestinal failure. Clin Nutr. 2013;32(6):983–987. | |
Seidner D. Parenteral nutrition-associated metabolic bone disease. JPEN J Parenter Enteral Nutr. 2002;26(5):S37–S42. | |
Cohen-Solal M, Baudoin C, Joly F, et al. Osteoporosis in patients on long-term home parenteral nutrition: a longitudinal study. J Bone Miner Res. 2003;18(11):1989–1994. | |
Perlmutter DH, Boyle JT, Campos JM, Egler JM, Watkins JM. D-lactic acidosis in children: an unusual metabolic complication of small bowel resection. J Pediatr. 1983;102(2):234–238. | |
Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin Gastroenterol Hepatol. 2006;4(1):11–20. | |
Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Disease Society of America. Clin Infect Dis. 2009;49(1):1–45. | |
Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2011;34(Suppl 1):S1–S110. | |
Dannenberg C, Bierbach U, Rothe A, et al. Ethanol-lock technique in the treatment of bloodstream infections in pediatric oncology patients with Broviac catheter. J Pediatr Hematol Oncol. 2003;25(8):616–621. | |
Metcalf S, Chambers S, Pithie A. Use of ethanol locks to prevent recurrent central line sepsis. J Infect. 2004;49(1):20–22. | |
Salvino R, Ghanbta R, Seidner D, Mascha E, Xu Y, Steiger E. Liver failure is uncommon in adults receiving long-term parenteral nutrition. JPEN J Parenter Enteral Nutr. 2006;30(3):202–208. | |
Cavicchi M, Crenn P, Beau P, Degott C, Boutron MC, Messing B. Severe liver complications associated with long-term parenteral nutrition are dependent on lipid parenteral input. Transplant Proc. 1998;30(6):2547. | |
Cavicchi M, Beau P, Crenn P, et al. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med. 2000;132(7):525–532. | |
Matarese LE, Steiger E. Dietary and medical management of short bowel syndrome in adult patients. J Clin Gastroenterol. 2006;40 Suppl 2:S85–S93. | |
Ching YA, Gunik K, Modi B, Jaksic T. Pediatric intestinal failure: nutrition, pharmacologic and surgical approaches. Nutr Clin Pract. 2007;22(6):653–663. | |
Nucci A, Burns RC, Armah T, et al. Interdisciplinary management of pediatric intestinal failure: a 10-year review of rehabilitation and transplantation. J Gastrointest Surg. 2008;12(3):429–435. | |
Byrne TA, Veglia L, Camelio M, et al. Beyond the prescription: optimizing the diet of patients with short bowel syndrome. Nutr Clin Pract. 2000;15(6):306–311. | |
Kelly DG, Nadeau J. Oral rehydration solution: a “low-tech” oft neglected therapy. Pract Gastroenterol. 2004;28(10):51–62. | |
Lennard-Jones JE. Oral rehydration solutions in short bowel syndrome. Clin Ther. 1990;12 Suppl A:129–137. | |
Winkler MF, Wetle T, Smith C, Hagan E, Maillet J, Touger-Decker R. The meaning of food and eating among home parenteral nutrition dependent adults with intestinal failure: a qualitative inquiry. J Am Diet Assoc. 2010;110(11):1676–1683. | |
Dsilna A, Christensson K, Alfredsson L, Lagercrantz H, Blennow M. Continuous feeding promotes gastrointestinal tolerance and growth in very low birth weight infants. J Pediatr. 2005;147(1):43–49. | |
Kumpf VJ. Pharmacologic management of diarrhea in patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014;38(Suppl 1):38S–44S. | |
Royall D, Wolever TM, Jeejeebhoy KN. Evidence for colonic conservation of malabsorbed carbohydrate in short bowel syndrome. Am J Gastroenterol. 1992;87(6):751–756. | |
O’Keefe SJH, Peterson ME, Fleming CR. Octreotide as an adjunct to home parenteral nutrition in the management of permanent end-jejunostomy. JPEN J Parenter Enteral Nutr. 1994;18(1):26–34. | |
Westergaard H. Bile acid malabsorption. Curr Treat Options Gastroenterol. 2007;10(1):28–33. | |
Quigley EM, Quera R. Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology. 2006;130(2 Suppl 1):S78–S90. | |
Jeppesen PB. Pharmacologic options for intestinal rehabilitation in patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014;38(Suppl 1):45S–52S. | |
Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60(7):902–914. | |
Winkler MF, Fujioka K, Youssef N, et al. Teduglutide enhances nutrient absorption in adult subjects with short bowel syndrome and maintains nutritional balance despite significant reductions in parenteral support. JPEN J Parenter Enteral Nutr. 2012;36(1):127–128. | |
Bianchi A. From the cradle to enteral autonomy: the role of autologous gastrointestinal reconstruction. Gastroenterology. 2006;130 Suppl 1:S138–S146. | |
Bianchi A. Intestinal loop lengthening – a technique for increasing small intestinal length. J Pediatr Surg. 1980;15(2):145–151. | |
Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial transverse enteroplasty (STEP):a novel bowel lengthening procedure. J Pediatr Surg. 2003;38(3):425–429. | |
Mercer DF, Iverson AK, Culwell KA. Nutrition and small bowel transplantation. Nutr Clin Pract. Epub June 19, 2014. | |
Matarese LE, Jeppesen PB, O’Keefe SJ. Short bowel syndrome in adults: the need for an interdisciplinary approach and coordinated care. JPEN J Parenter Enteral Nutr. 2014;38(Suppl 1):60S–64S. | |
Khalil BA, Ba’ath M, Aziz A, et al. Intestinal rehabilitation and bowel reconstructive surgery: improved outcomes in children with short bowel syndrome. J Pediatr Gastroenterol. 2012;54(4):505–509. | |
Ayers P, Holcombe B, Plogsted S, Guenter P. ASPEN Parenteral Nutrition Handbook. 2nd ed. Silver Spring (MD):American Society for Parenteral and Enteral Nutrition, 2014:123–125. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.