Back to Journals » Drug Design, Development and Therapy » Volume 9
Nutrigenomic effects of edible bird’s nest on insulin signaling in ovariectomized rats
Authors Hou Z, Umar Imam M , Ismail M, Ooi DJ, Ideris A, Mahmud R
Received 11 January 2015
Accepted for publication 11 February 2015
Published 14 August 2015 Volume 2015:9 Pages 4115—4125
DOI https://doi.org/10.2147/DDDT.S80743
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Zhiping Hou,1,2 Mustapha Umar Imam,1 Maznah Ismail,1,3 Der Jiun Ooi,1 Aini Ideris,4 Rozi Mahmud5
1Laboratory of Molecular Biomedicine, Institute of Bioscience, Universiti Putra Malaysia, Serdang, Malaysia; 2Department of Pathology, Chengde Medical University, Chengde, People’s Republic of China; 3Department of Nutrition and Dietetics, Universiti Putra Malaysia, Serdang, Malaysia; 4Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang, Malaysia; 5Department of Imaging, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia
Abstract: Estrogen deficiency alters quality of life during menopause. Hormone replacement therapy has been used to improve quality of life and prevent complications, but side effects limit its use. In this study, we evaluated the use of edible bird’s nest (EBN) for prevention of cardiometabolic problems in rats with ovariectomy-induced menopause. Ovariectomized female rats were fed for 12 weeks with normal rat chow, EBN, or estrogen and compared with normal non-ovariectomized rats. Metabolic indices (insulin, estrogen, superoxide dismutase, malondialdehyde, oral glucose tolerance test, and lipid profile) were measured at the end of the experiment from serum and liver tissue homogenate, and transcriptional levels of hepatic insulin signaling genes were measured. The results showed that ovariectomy worsened metabolic indices and disrupted the normal transcriptional pattern of hepatic insulin signaling genes. EBN improved the metabolic indices and also produced transcriptional changes in hepatic insulin signaling genes that tended toward enhanced insulin sensitivity, and glucose and lipid homeostasis, even better than estrogen. The data suggest that EBN could meliorate estrogen deficiency-associated increase in risk of cardiometabolic disease in rats, and may in fact be useful as a functional food for the prevention of such a problem in humans. The clinical validity of these findings is worth studying further.
Keywords: ovariectomy, lipid metabolism, insulin resistance, antioxidant, aging
Introduction
Menopause is characterized by depletion of the ovarian follicles and decreasing levels of estrogen and related hormones. As menopause sets in, the ovaries progressively fail to produce estrogen, resulting in symptoms that may be severe and disabling and have an impact on quality of life.1 Estrogen has modulatory effects on metabolic processes including the maintenance of optimal glucose and lipid homeostasis, which it does through regulation of key hormones and genes involved in cardiometabolic health.2 During menopause, fluctuations and/or relative or absolute deficiency of estrogen and other sex hormones will result in loss of the balance maintained by these hormones, thereby leading to a disturbed internal milieu and heightened risk of diseases including insulin resistance and associated problems.3,4
The symptoms and diseases that are a consequence of menopause have received considerable attention, due to their distressing nature and impact on quality of life. It is increasingly becoming a concern for women because of increasing life expectancy. Hormone replacement therapy has been used to restore premenopausal hormonal levels in order to reverse menopausal problems, but associated side effects including cancers and cardiovascular disease have necessitated the search for alternatives.5,6 Phytoestrogens from plants have also been associated with side effects similar to those of hormone replacement therapy.7 Alternatives like Remifemin® have not offered significant improvements in long-term sequelae of menopause like cancer and cardiometabolic disease risk, and side effects further limit their use.8
The burden of cardiometabolic disease has grown considerably over the years, and in women the risk of these diseases increases significantly after menopause.3,4 Edible bird’s nest (EBN) has been used as a traditional supplement mostly by Asians to improve wellbeing.9 Recent evidence indicates that EBN may have anti-inflammatory and antioxidative properties,10–12 which can be beneficial in cardiometabolic disease.13,14 In this study, we determined the effects of EBN on cardiometabolic indices in ovariectomized rats, and a possible mechanistic basis for such effects. We hypothesized that the outcome of the study could provide the evidence needed for use of EBN as a functional food in preventing cardiometabolic disease in menopause, where the risk of such is increased due to loss of protection from estrogen and related hormones.
Materials and methods
Materials
A rat insulin enzyme-linked immunosorbent assay kit was purchased from Millipore (Billerica, MA, USA), while kits for superoxide dismutase (SOD) and estrogen were from Cell Biolabs (Cell Biolabs Inc, San Diego, CA, USA) and Cusabio Biotech Co Ltd (Wuhan, People’s Republic of China), respectively. The GenomeLab™ GeXP start kit was from Beckman Coulter Inc (Miami, FL, USA) and the RNA extraction kit was from RBC Bioscience Corp (Taipei, Taiwan). MgCl2 and DNA Taq polymerase were purchased from Thermo Fisher Scientific (Pittsburgh, PA, USA), while RCL2 solution was purchased from Alphelys (Toulouse, France). Glucometer strips were from Roche Diagnostics (Indianapolis, IN, USA) and lipid profile kits (low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, total cholesterol, and triglycerides) were purchased from Randox Laboratories Ltd (Crumlin, County Antrim, UK). Rat chow was obtained from Specialty Feeds (Glen Forrest, WA, Australia). Ketamine/xylazine was from Sigma Chemical Co (St Louis, MO, USA) and other solvents of analytical grade were purchased from Merck (Darmstadt, Germany).
EBN samples
Ready-to-use EBN was supplied by Niah Bird’s Nest Trading Company (Sarawak, Malaysia), and was incorporated into standard rat chow for animal feeding. For determination of nutritional composition, EBN was dried at 50°C for 3 days, ground into powder, and used. Nutritional composition (protein, carbohydrate, fat, ash, and moisture; Table 1) was determined as reported previously.15
  | Table 1 Nutritional values for edible bird’s nest |
Animal handling and feeding
Approval for use of animals was granted by the Animal Care and Use Committee of the Faculty of Medicine and Health Sciences, Universiti Putra Malaysia (approval UPM/IACUC/AUP-R012/2014), and the animals were handled as stipulated by the guidelines for the use of animals. Thirty-six Sprague-Dawley rats (3 months old, female, 160–180 g) were housed under controlled conditions (12-hour light/12-hour dark cycle, 20°C–22°C, 40%–50% humidity) with free access to water and food for 2 weeks prior to the experiments. After acclimatization, rats were ovariectomized under anesthesia using 10 mg/60 mg/kg xylazine/ketamine (intraperitoneally), except for the normal controls. The rats were then observed for 4 weeks and randomly assigned to one of five groups (n=6): a sham group, maintained on standard rat chow; an ovariectomy (OVX) control group maintained on standard rat chow; an OVX + estrogen group maintained on standard rat chow and daily estrogen (0.2 mg/kg body weight); an OVX + 3% EBN group maintained on standard rat chow containing 3% EBN; and an OVX + 1.5% EBN group maintained on standard rat chow containing 1.5% EBN. The normal group was not ovariectomized and was maintained on standard rat chow. Treatments lasted for 12 weeks, and food intake in each group was adjusted to the average intake each day according to observation of the OVX group the day before. Weights were measured weekly, and the total amount of feed (g) given was reviewed weekly based on the weekly weights of the rats. At the end of the experiment, all animals were exsanguinated after anesthesia (10 mg/60 mg/kg xylazine/ketamine, intraperitoneally). The livers were collected into RCL2 solution for gene expression studies.
OGTT and lipid profile
At the end of the intervention, an oral glucose tolerance test (OGTT) was performed on each animal after 12 hours of an overnight fast, and measurements were taken with a glucometer. Serum samples from blood collected on the day of euthanasia were used to analyze the rat lipid profiles (total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides) using a Dimension Xpand Plus Integrated chemistry system (Siemens, Germany) with commercially available kits.
Determination of serum estrogen and insulin
Serum samples were used for measurements of estrogen and insulin using the respective enzyme-linked immunosorbent assay kits according to the manufacturers’ instructions. Absorbance was read on a microplate reader (BioTek Synergy H1 Hybrid Reader, BioTek Instruments Inc, Winooski, VT, USA) and results were calculated from the respective standard curves: estrogen (y = −30.12x +113.73, R2=1), insulin (y =0.762x −0.143, R2=0.966). Additionally, homeostatic model assessment of insulin resistance (HOMA-IR), a measure of insulin sensitivity, was computed from the fasting plasma glucose and insulin levels as reported previously.16
Hepatic antioxidative markers
Superoxide dismutase enzyme
Liver homogenates were used for SOD quantification using the enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions. Absorbance were read on a microplate reader (BioTek Synergy H1 Hybrid Reader) and results were calculated from the standard curve (y =0.143x −0.017, R2=1).
Thiobarbituric acid reactive substances assay
Thiobarbituric acid reactive substances were determined using a modified method based on the protocol of Chan et al.17 Briefly, homogenized liver tissues (50 mg/100 μL phosphate-buffered saline) were added to 0.25 N HCl, 15% trichloroacetic acid, and 0.375% thiobarbituric acid separately, then incubated at 100°C for 10 minutes, and centrifuged at 3,000 rpm for 15 minutes. Finally, absorbance of the supernatant were read at 540 nm using the Synergy H1 Hybrid Multi-Mode microplate reader. Malondialdehyde (MDA) was used as the standard (y =0.1982x −0.1898, R2=0.9947).
RNA extraction, reverse transcription, and multiplex polymerase chain reaction analyses
RNA was extracted from rat livers using the Total RNA isolation kit according to the manufacturer’s instructions. Primer sequences were designed on the National Center for Biotechnology Information website, except for the internal control (KanR), which was supplied by Beckman Coulter. The primers (Table 2) were supplied by Integrated DNA Technologies (Singapore), and reconstituted in 1× TE buffer according to the protocol outlined in the GenomeLab GeXP kit. Reverse transcription and polymerase chain reaction were performed according to the GenomeLab GeXP kit protocol in an XP Thermal Cycler (Bioer Technology, Germany). The polymerase chain reaction products were finally analyzed with a GeXP genetic analysis system, and the results were normalized using eXpress Profiler software based on the manufacturer’s instructions.
Statistical analysis
Statistical analyses were done using one-way analysis of variance with Tukey’s honest significant difference test. The data are expressed as the mean ± standard error of the mean. P<0.05 indicates statistical significance. All values were statistically evaluated using Statistical Package for the Social Sciences version 20.0 (SPSS Inc, Chicago, IL, USA).
Results and discussion
Food intake and body weight
Menopause is characterized by a drop in estrogen levels, which results in lipid and glucose metabolic perturbation.1,3,4 In this study, serum estrogen levels in the OVX group were significantly lower than in the other groups (Table 3), suggesting that removal of the ovaries produced a condition similar to menopause in the rats. The higher levels in the estrogen group could be attributed to exogenous estrogen administration, while the high levels of the hormone in the EBN group could have been due to increased extragonadal synthesis of estrogen18 induced by EBN or possibly estrogen-like compounds absorbed from EBN. Body weights were similar for all groups at the start of the experiment (Table 3), and calorie intake remained similar throughout the intervention period. At the end, however, differences in weight gain were observed. The OVX group had the highest weight gain, indicating that loss of estrogen affected body fat deposition and distribution. Moreover, estrogen is believed to regulate weight in women, while menopause is associated with increased weight gain, which is linked to increased risk of cardiometabolic disease.2–4 Estrogen treatment and EBN produced lower weight gains similar to the control group, suggesting that both treatments had some weight-modulating properties, possibly mediated through estrogen and/or estrogen-mimetic effects.
Serum lipid profile
Table 4 shows the lipid profiles for the different groups. Total cholesterol for the OVX group was highest although no significant differences were observed between the groups. Low-density lipoprotein cholesterol was also highest in the OVX group, while the others were significantly lower, except for the 1.5% EBN group. Triglyceride levels were significantly lower in the EBN groups in comparison with the OVX group, while high-density lipoprotein cholesterol levels were not significantly different between the groups. As expected, estrogen improved the lipid profiles. Estrogen modulates cholesterol metabolism, and in estrogen-deficient states similar to OVX, there is dysregulation of cholesterol metabolism in favor of higher cholesterol levels, with an increased risk of cardiometabolic disease.4 EBN on the other hand was able to ameliorate lipid profile and cholesterol ratios (Table 4) similar to estrogen. This effect of EBN on the lipid profile may have been secondary to the enhanced estrogen concentration induced by EBN, but other EBN constituents may also have played a role since EBN is known to be bioactive-rich.9 The improved lipid profiles observed in this study suggest that EBN could lower the risk of cardiometabolic disease without the associated risks of estrogen administration.
OGTT, serum insulin, and HOMA-IR
The OGTT results are shown in Figure 1. The glycemic response in the OVX group was impaired in comparison with the other groups, which showed similar and significantly (P<0.05) lower responses (Figure 1A). Moreover, the area under the curve for glucose over 120 minutes (Figure 1B) was significantly lower (P<0.05) for the EBN and estrogen treatments in comparison with the OVX group. Additionally, the insulin level was highest in the OVX group, which could have been a counter-response to the loss of estrogen-regulated insulin action in the rats (Figure 2). The estrogen group, on the other hand, showed significantly lower insulin levels (P<0.05), while the EBN groups were not significantly different from the OVX group. HOMA-IR results showed that the OVX group had the highest tendency for insulin resistance, while estrogen therapy significantly improved insulin sensitivity (P<0.05). These effects of OVX and estrogen therapy mirror what has been reported previously;4 OVX tends to increase the risk of insulin resistance, which can be prevented by estrogen therapy. EBN treatments showed dose-dependent effects on insulin sensitivity, but only the 3% EBN treatment showed significantly better results than those seen in the OVX group. Although estrogen may prevent the OVX-induced risk of insulin resistance as demonstrated in the present study (lower HOMA-IR in the estrogen group), EBN may be preferred since its consumption is not associated with side effects as with estrogen therapy, except when it is adulterated.9
Hepatic antioxidant capacity of EBN
Oxidative stress is increased while antioxidants are decreased in estrogen-deficient states. This imbalance is thought to underlie some problems related to menopause.19 Thus, in this study, MDA levels, indicative of oxidative stress, were potentiated in the OVX group while SOD, an antioxidant enzyme, was suppressed (Figure 3), in keeping with a report by Muthusami et al.20 Further, estrogen treatment reduced MDA and improved SOD levels but not as well as EBN. Moreover, the side effects of estrogen therapy may be associated with the pro-oxidant effects of exogenous estrogen.21 EBN significantly ameliorated oxidative stress status and improved antioxidant abilities, in agreement with reports on its antioxidant properties.11,12 The results therefore suggest that improved antioxidant enzymes may partly form the basis for improved metabolic indices in ovariectomized rats fed EBN, since improved antioxidant status has been linked with better metabolic outcomes.22
mRNA levels of hepatic insulin signaling genes
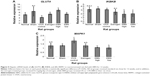
The results thus far indicate that EBN could lower the risk of estrogen deficiency-induced perturbations in glucose and lipid homeostasis. In an effort to understand some of the mechanistic bases for the observed effects, we evaluated mRNA levels for hepatic insulin signaling genes (Figures 4–6). Figure 4 shows the effects of the interventions on mRNA levels of the hepatic insulin receptor, insulin receptor substrate 2 (IRS2), and phosphoinositide-3-kinase (PI3K). Insulin exerts its effects after binding to its receptor and activating a cascade of events mediated by IRS2,23 in which PI3K plays a prominent role through enhanced insulin signaling in the cell.24 Disruption of this pathway promotes insulin resistance. In this study, OVX downregulated IRS2 and PI3K, suggesting that estrogen deficiency-induced interruption of cellular insulin signaling contributed to worsening of glucose and lipid homeostasis in the rats. Interestingly, EBN increased expression of the insulin receptor, IRS2, and PI3K even better than estrogen therapy, indicating that its ability to prevent estrogen deficiency-induced worsening of glucose and lipid homeostasis in ovariectomized rats was partly through transcriptional upregulation of insulin signaling.
Furthermore, OVX upregulated mitogen-activated protein kinase (MAPK) and inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta (IKBKB, Figure 5), which have both been implicated in impaired insulin signaling.25,26 These genes have been reported to influence the activity of IRS2, and the OVX-induced low transcriptional levels of IRS2 observed in this study may have been due to upregulation of MAPK1 and IKBKB.25,26 The EBN groups had lower mRNA levels of these genes in comparison with other groups, further suggesting that the effects of EBN on transcriptional regulation of IRS2 may have been through MAPK1 and IKBKB. Glucose transporter type 4 (GLUT4) transports glucose into cells for metabolism and is downregulated in insulin-resistant states.27 Although the exact contribution of hepatic expression of GLUT4 to glucose transport remains a matter of debate, there are indications that its dysregulation is linked to insulin resistance.28 In this study, OVX downregulated GLUT4, while EBN treatments upregulated the gene in a dose-dependent manner. Moreover, lower levels of MAPK, with consequently higher PI3K levels, have been reported to enhance glucose uptake through increased GLUT4 expression.27 In keeping with findings of decreased MAPK and increased PI3K and GLUT4 in this study.
Transcriptional levels of other genes that contribute to enhanced cellular glucose sensing and homeostasis (glucokinase, pyruvate kinase-liver isoform, and potassium inwardly rectifying channel, subfamily J, member 11 [KCNJ11] genes) were downregulated in the OVX group (Figure 6). Conversely, the EBN group showed increased expression of these genes, except KCNJ11, even better than estrogen therapy. The implications of these effects could be increased hepatic glucose storage and utilization (glucokinase and pyruvate kinase-liver isoform),29 which may then enhance cellular adenosine triphosphate levels and subsequent upregulation of KCNJ11 with improved glucose homeostasis.30
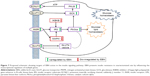
Based on the effects of EBN observed in this study, we propose that EBN transcriptionally regulates multiple targets in the insulin signaling pathway (Figure 7) and that this is the basis for the improved insulin signaling and glucose and lipid homeostasis.
Overall, the data show that OVX worsened glucose and lipid homeostasis and oxidative stress (low insulin and SOD levels; high body weight, HOMA-IR, MDA, and lipid profile levels; and impaired insulin signaling) in rats. Estrogen therapy, on the other hand, was able to improve the lipid profile and OGTT, SOD, and MDA levels, and was even associated with some transcriptional changes in hepatic insulin signaling genes that tended toward better glucose and lipid homeostasis. However, EBN improved glucose and lipid homeostasis, lowered weight and oxidative stress, and enhanced estrogen deficiency-induced impaired signaling of insulin better than estrogen. Simple linear regression analyses indicate that estrogen levels significantly correlated with changes in HOMA-IR (r= −0.82, P=0.046), SOD (r=0.82, P=0.046), MDA (r= −0.87, P=0.023), and PI3K-mediated insulin signaling (r=0.86, P=0.027). Additionally, SOD levels (r= −0.86, P=0.028) and PI3K-mediated insulin signaling (r= −0.85, P=0.033), but not MDA levels (r=0.53, P=0.28) correlated with HOMA-IR in this study. Multiple linear regression analyses indicate further that changes in estrogen were overall strong determinants of changes in SOD, MDA, HOMA-IR, and PI3K expression changes (R=1, R2=0.995), and that estrogen, SOD, MDA, and PI3K expression was a significant predictor of HOMA-IR (R=0.987, R2=0.873). The combined effects of EBN on these markers of cardiometabolic disease, coupled with the known side effects of estrogen therapy, suggest that EBN may be better than hormone replacement therapy at preventing menopause-associated cardiometabolic disease, especially impaired glucose and lipid homeostasis.
Conclusion
OVX promotes metabolic perturbations that may give rise to cardiometabolic disease, and although the use of estrogen may offer some protection against some of these cardiometabolic problems, as suggested by the present data, use of estrogen therapy is limited by side effects. EBN has demonstrated an ability to improve cardiometabolic indices and enhance transcriptional regulation of insulin signaling genes even better than estrogen. These results demonstrate for the first time the potential for EBN to be used as a functional food for prevention of cardiometabolic disease associated with estrogen deficiency. These findings are worth studying further in humans to ascertain the clinical validity of EBN in preventing these diseases.
Acknowledgments
The authors acknowledge the financial support of the Ministry of Science, Technology and Innovation, the e-ScienceFund, Malaysia (vote 5450666); and the staff of the Laboratory of Molecular Biomedicine for their assistance during the study.
Disclosure
The authors report no conflicts of interest in this work.
References
Hess R, Thurston RC, Hays RD, et al. The impact of menopause on health-related quality of life: results from the STRIDE longitudinal study. Qual Life Res. 2012;21(3):535–544. | ||
Louet JF, Cedric LM, Franck MJ. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004;6(3):180–185. | ||
Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol. 2009;5(10):553–558. | ||
Gaspard UJ, Gottal JM, van den Brûle FA. Postmenopausal changes of lipid and glucose metabolism: a review of their main aspects. Maturitas. 1995;21(3):171–178. | ||
Beral V, Banks E, Reeves G. Evidence from randomised trials on the long-term effects of hormone replacement therapy. Lancet. 2002; 360(9337):942–944. | ||
Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(7):872–881. | ||
Tempfer CB, Froese G, Heinze G, Bentz E-K, Hefler L-A, Huber JC. Side effects of phytoestrogens: a meta-analysis of randomized trials. Am J Med. 2009;122(10):939–946. | ||
Nedrow A, Miller J, Walker M, Nygren P, Huffman LH, Nelson HD. Complementary and alternative therapies for the management of menopause-related symptoms: a systematic evidence review. Arch Intern Med. 2006;166(16):1453–1465. | ||
Marcone MF. Characterization of the edible bird’s nest the “Caviar of the East”. Food Res Int. 2005;38(10):1125–1134. | ||
Vimala B, Hussain H, Nazaimoon WW. Effects of edible bird’s nest on tumour necrosis factor-alpha secretion, nitric oxide production and cell viability of lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Agric Immunol. 2012;23(4):303–314. | ||
Yew MY, Koh RY, Chye SM, Othman I, Ng KY. Edible bird’s nest ameliorates oxidative stress-induced apoptosis in SH-SY5Y human neuroblastoma cells. BMC Complement Altern Med. 2014;14(1):391. | ||
Yida Z, Imam MU, Ismail M. In vitro bioaccessibility and antioxidant properties of edible bird’s nest following simulated human gastro-intestinal digestion. BMC Complement Altern Med. 2014;14(1):468. | ||
Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84(21):705–712. | ||
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. | ||
Imam MU, Ishaka A, Der Juin O, et al. Germinated brown rice regulates hepatic cholesterol metabolism and cardiovascular disease risk in hypercholesterolemic rats. J Funct Foods. 2014;8:193–203. | ||
Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008;295(5): 1269–1276. | ||
Chan KW, Khong NM, Iqbal S, Ch’ng SE, Babji AS. Preparation of clove buds deodorized aqueous extract (CDAE) and evaluation of its potential to improve oxidative stability of chicken meatballs in comparison to synthetic and natural food antioxidants. J Food Qual. 2012; 35(3):190–199. | ||
Ye L, Chan MY, Leung LK. The soy isoflavone genistein induces estrogen synthesis in an extragonadal pathway. Mol Cell Endocrinol. 2009;302(1):73–80. | ||
Sánchez-Rodríguez MA, Zacarías-Flores M, Arronte-Rosales A, Correa-Muñoz E, Mendoza-Núñez VM. Menopause as risk factor for oxidative stress. Menopause. 2012;19(3):361–367. | ||
Muthusami S, Ramachandran I, Muthusamy B. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta. 2005;360(1):81–86. | ||
Bhat HK, Calaf G, Hei TK, Loya T, Vadgama JV. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc Natl Acad Sci U S A. 2003;100(7):3913–3918. | ||
Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59(7):365–373. | ||
Brady MJ. IRS2 takes center stage in the development of type 2 diabetes. J Clin Invest. 2004;114(7):886–888. | ||
Hirsch E, Costa C, Ciraolo E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J Endocrinol. 2007;194(2): 243–256. | ||
Igarashi M, Wakasaki H, Takahara N, et al. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest. 1999;103(2):185–195. | ||
Arkan MC, Hevener AL, Greten FR, et al. IKK links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11(2):191–198. | ||
Carlson CJ, Koterski S, Sciotti RJ, Poccard GB, Rondinone CM. Enhanced basal activation of mitogen-activated protein kinases in adipocytes from type 2 diabetes potential role of p38 in the downregulation of GLUT4 expression. Diabetes. 2003;52(3):634–641. | ||
Karim S, Adams DH, Lalor PF. Hepatic expression and cellular distribution of the glucose transporter family. World J Gastroenterol. 2012; 18(46):6771–6781. | ||
Lenzen S. A fresh view of glycolysis and glucokinase regulation: history and current status. J Biol Chem. 2014;289(18):12189–12194. | ||
Proks P, Girard C, Ashcroft FM. Functional effects of KCNJ11 mutations causing neonatal diabetes: enhanced activation by Mg ATP. Hum Mol Genet. 2005;14(18):2717–2726. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.