Back to Archived Journals » Neuroscience and Neuroeconomics » Volume 3
Neural correlates of fear: insights from neuroimaging
Authors Garfinkel S, Critchley H
Received 6 May 2014
Accepted for publication 19 June 2014
Published 1 December 2014 Volume 2014:3 Pages 111—125
DOI https://doi.org/10.2147/NAN.S35915
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Sarah N Garfinkel,1,2 Hugo D Critchley1,2
1Sackler Centre for Consciousness Science, 2Department of Psychiatry, Brighton and Sussex Medical School, University of Sussex, Brighton, UK
Abstract: Fear anticipates a challenge to one's well-being and is a reaction to the risk of harm. The expression of fear in the individual is a constellation of physiological, behavioral, cognitive, and experiential responses. Fear indicates risk and will guide adaptive behavior, yet fear is also fundamental to the symptomatology of most psychiatric disorders. Neuroimaging studies of normal and abnormal fear in humans extend knowledge gained from animal experiments. Neuroimaging permits the empirical evaluation of theory (emotions as response tendencies, mental states, and valence and arousal dimensions), and improves our understanding of the mechanisms of how fear is controlled by both cognitive processes and bodily states. Within the human brain, fear engages a set of regions that include insula and anterior cingulate cortices, the amygdala, and dorsal brain-stem centers, such as periaqueductal gray matter. This same fear matrix is also implicated in attentional orienting, mental planning, interoceptive mapping, bodily feelings, novelty and motivational learning, behavioral prioritization, and the control of autonomic arousal. The stereotyped expression of fear can thus be viewed as a special construction from combinations of these processes. An important motivator for understanding neural fear mechanisms is the debilitating clinical expression of anxiety. Neuroimaging studies of anxiety patients highlight the role of learning and memory in pathological fear. Posttraumatic stress disorder is further distinguished by impairment in cognitive control and contextual memory. These processes ultimately need to be targeted for symptomatic recovery. Neuroscientific knowledge of fear has broader relevance to understanding human and societal behavior. As yet, only some of the insights into fear, anxiety, and avoidance at the individual level extrapolate to groups and populations and can be meaningfully applied to economics, prejudice, and politics. Fear is ultimately a contagious social emotion.
Keywords: amygdala, anxiety, arousal, autonomic, emotion, phobia
Emotions and fear
Emotions can be viewed as transient stereotyped reactions to motivationally salient events or stimuli. These reactions reprioritize perceptual, cognitive, and behavioral states. Emotions have psychological (cognitive, perceptual, and experiential), physiological (bodily arousal), and behavioral (expressions and action tendencies) dimensions. Most formulations of emotion also emphasize a social role, where such motivational states as hunger and thirst are conceptualized at a lower level. Distinctions based on temporal duration are made between emotions and other affective states, eg, moods. Some researchers define emotions as the (bodily) responses, as distinct from feeling states that arise as the perceptual/experiential consequences of the emotion.1 Many authorities2–4 propose the notion of basic emotions: each emotion type (eg, happiness, disgust, anger, and fear) is envisaged as distinct, with separate links to evolutionary imperatives. Therefore, fear is proposed as a distinct emotion characterized by an orientation of resources toward physical self-preservation and protection. It carries the anticipation of a potential catastrophic outcome that challenges the integrity and viability of an individual. Fear encompasses characteristic negative psychological feelings and states of mental and physiological arousal. Fear responses can be engendered rapidly and preconsciously to impact on behavior, attention, and memory processes. There are a plethora of close and distant fear signals across the senses for the rapid communication of fear to others, notably facial expression (wide eyes5), vocalizations (gasps toward screams), posture (retracted), and skin (pallor, piloerection) are potent cues for conveying the presence of danger. These signals even communicate across species.6 Nevertheless, the view of fear as a primary emotion can break down with detailed analysis. Perhaps more accurately, fear can be conceptualized as an overarching emotion category that embraces different but related instances and functions.7–9
Outline
In this review, we take an integrative perspective on what we can learn from neuroimaging studies of fear, and how such studies examining mechanisms at the level of the individual implicitly inform our understanding of social affective processes and potentially have broader relevance for society. Specifically, this review addresses the expression and measurement of fear, and the different ways to induce and assess fear, including fear-induction paradigms, fear conditioning (focusing on fear acquisition, extinction learning), and threat anticipation to cues and contexts. The impact of fear on cognitive functioning is considered in sections pertaining to attention and memory. The contextual modulation of fear processing is also highlighted, since the magnitude and expression of responses to threat are sensitive to both the internal (psychological and physiological) state of the individual and/or external factors (environment, social context, and proximity of threat). A section examines pharmacological and genetic imaging describing neurochemical modulation and individual differences (genetic or otherwise) in anxiety and fear processing. This leads to clinical expressions of fear, notably in posttraumatic stress disorder (PTSD) and phobia, where neuroimaging reveals neural substrates underlying fear psychopathology and potential strategies for intervention. We focus on fear mechanisms within the individual and how fear signals are exchanged between individuals. These processes underpin and are expressions of genetic and societal processes that transcend the individual. The topic of human fear, while a focus of much interest a decade ago, has not been comprehensively reviewed within the recent literature, despite important new empirical findings. This review paper provides a much-needed updated perspective on human fear, for which improved understanding has broader implications across and beyond basic, clinical, and social psychology.
Expression and measurement of fear
The feeling states associated with fear and anxiety have a substantial physiological/interoceptive component. This is often cited as the basis of the peripheral theories of emotion, illustrated by the suggestion of William James that we feel afraid because we run from a bear.10 Emotional feeling states are proposed to originate from sensory feedback representation of changes induced within the body by emotional reaction. Such feelings are typically viewed as valenced, but poorly localizable mental phenomena. However, fear feelings are physiological responses, and are often clear and localizable: these include a tightness in the throat, tension in the chest, dry mouth, sweating, gastrointestinal sensations, and heart pounding. Such sensations are instilled with a feeling of danger, imminent collapse, or catastrophe. While the mental experience of fear is tied to future uncertainties, it is intensified by physiological sensations and their perceived negative meaning.
The pattern of autonomic bodily response elicited by fear induction is characterized by heightened sympathetic activation, reflected in increased heart rate, myocardial contractility, peripheral vasoconstriction, and increased electrodermal activity.11 In contrast to anger, systemic vascular resistance is often reduced.12,13 Parasympathetic vagal influences on the heart are also usually diminished, decreasing heart-rate variability.14 In some fear contexts, however, bradycardia appears as a dominant and amplified early orienting response that enhances cardiac filling for the next heartbeat. A sudden display of threat cues can elicit this initial bradycardia,15 frequently accompanied by motoric freezing. Both bradycardia and freezing are proposed to enhance information intake and the appraisal of the source of threat. Tachycardia follows shortly after, yet increasing vagal tone in anticipation of an imminent threat outcome may lead to complex triphasic cardiac fear responses. Here, the proximity of threat is an important determinant of the pattern of heart-rate response.16 Fear states are also accompanied by increased respiratory rate as a result of reduced duration of expiration and more variable inspiratory flow, which can decrease blood carbon dioxide (CO2) levels.12,14,17 Fear also elicits humoral (adrenal) stress responses, with adrenaline and cortisol release into the bloodstream.18 Within neuroimaging contexts, the objective autonomic measurement of fear responses is mostly confined to unitary autonomic measures of arousal, notably electrodermal responses, particularly in the context of conditioned learning.19–22 The coupling of amygdala responses to evoked autonomic changes appears a useful signature of fear.23,24 Other brain regions may be more important to sympathetic (electrodermal or cardiac) arousal in nonthreat contexts.25 Cortisol responses are more often used in stress-induction studies (often including social threat challenges).18
Fear reactions
Fear reactions include visible changes in bodily and facial expression, some of which relate directly to sympathetic autonomic reactions. Widening of the eyes, gaping of the mouth, and related changes in facial expression constitute automatic motor responses that can be measured even covertly using electromyography.26 These cues, rapidly perceived by others, along with more explicit signals of threat and distress (eg, screams), are potent drivers for the rapid spread of fear between individuals. Whole-body retraction is a component of early freezing and alarm response, linked to the “play dead” responses of animals (which may have a further human homologue in simple emotional fainting). Human correlates of freezing can be induced by external threat stimuli, yet can also be elicited by direct physiological induction of the fear states, eg, using 20% CO2 inhalation.27 Immobility is thus one basic early response to threat that is also expressed as impairments in behavioral measures, eg, slowed reaction time. In neuroimaging and other experimental contexts, delayed reaction times may be a useful objective index of fear, but again this is contextual: Fear-evoked enhancement of motor response (to flee or fight) is also associated with more rapid reaction times. These potentially conflicting inhibitory and facilitatory motor responses to threat are important, since they may show task dependence, change over the course of an experimental task, and show interindividual differences in their expression.
The startle reflex is a simple defensive reflex, typically to loud unexpected sounds. While animal studies typically measure whole-body startle responses, the focus in humans is on the blink component.28 The presence of a threat, often in the form of a fear-conditioned stimulus, results in an amplification of the startle response. The fear-potentiated startle response in animals is a reliable measure of central fear processing and used to test the likely efficacy of anxiolytic drugs. Fear-potentiated startle has been used in human neuroimaging experiments,29 yet its use in functional magnetic resonance imaging (fMRI) is constrained by logistical considerations.
Fear thus engages the individual systemically, changing the bodily state in a coordinated way that facilitates rapid adaptive responses to threat. These responses in the body are automatic, and can act as objective measures of threat processing that can then be related to self-reported fear and behavioral response. However, the dichotomy between fight and flight tendencies is mirrored in bodily response, where bradycardia or tachycardia and immobilization or mobilization may represent context- and experience-dependent expressions of fear.
Induction of fear in an individual
We can understand more about the expression and neural mechanisms of fear by directly evoking fear in individuals undergoing brain-imaging experiments. Ways by which fear can be induced directly within an individual include physiological challenge, pharmacological manipulation, direct exposure to intrinsically threatening stimuli (phobic stimuli, snakes, etc), threat learning, and psychological induction techniques (recollection/visualization). Perhaps more striking, though, is the contagion of fear responses between individuals, where fear signals (see later) in another’s voice, facial expression, and even smell30 can rapidly activate one’s own fear circuitry.
A raised concentration of CO2 in inhaled air or within the bloodstream is a potent physiological stimulus for inducing anxiety, fear, and panic. Such hypercapnia evokes a sensation of breathlessness, labeled “air hunger”. Widespread activation of cortical and subcortical regions is observed when CO2 levels are raised.31–33 There is enhanced engagement of the brain stem (pons), midbrain (including periaqueductal gray), hypothalamus, thalamus, amygdala and periamygdaloid regions, cingulate, anterior insula, caudate nuclei, and fusiform gyrus. Importantly for interpretation, such effects can be dissociated from generalized global effects of CO2 level on brain blood flow. Correspondingly, deactivations are observed within regions including the posterior cingulate and prefrontal cortex.31 Air hunger, even when CO2 levels are kept constant, elicits a similar pattern of neural activity responses, particularly involving the anterior insula cortex.33 Interestingly, CO2 inhalation can induce panic and fear sensations even in people with no functional amygdalae, challenging the view that the amygdala is necessary or sufficient for the experience of fear at the individual level, yet consistent with core contributions of brain-stem and insula regions in the generation of fear sensations.32,34 The novelty of this observation, to the patients concerned as well as to the scientific community, also highlights the fact that fear is almost always perceived in social contexts that likely necessitate amygdala involvement.
Among pharmacological agents associated with the induction of fear and panic, cholecystokinin tetrapeptide (CCK4) is a useful tool that has been employed in neuroimaging settings.35,36 CCK4 infusion can induce a reliable physiological and psychological replication of panic, which can be reversed with anxiolytic drugs, such as benzodiazepines. When administered in an fMRI experimental context, CCK4 activates the insula and cingulate cortices, temporal poles, thalamus, and cerebellar vermis.36 CCK4-induced panic also engages the ventral anterior cingulate cortex, lateral prefrontal regions, and precuneus, with around half of participants also showing amygdala-activity changes.35 Interestingly, CCK4 panic and its association with cingulate and insula activation may be relatively resistant to pharmacological suppression by potent anxiolytic benzodiazepines.37
Exposure to fear-inducing stimuli, threat of pain (see the “Fear learning and extinction” section), and recollection of previous threat, have all been used in neuroimaging contexts for the experimental provocation of fear and anxiety symptoms at the individual level. In humans, tasks that tap into social fears, including the threat of evaluation by others and anxiety about performing poorly in front of one’s peers, are often more potent stressors. Social evaluative threat with the anticipation of public scrutiny and related performance challenges, as evoked by the Trier social stress test,21,38–40 provide useful experimental models of everyday fears and anxiety for which neural processing overlaps with core features of “basic” fear. Across these different methods of fear induction, there is a reliable enhancement of activity within the dorsal anterior cingulate cortex, bilateral insula cortex, and (perhaps less consistently) subcortical activation within centers that include the amygdala, head of caudate, and brain stem (typically dorsal pons). Brain regions, including ventromedial orbital and subgenual cingulate cortices, are typically deactivated. Interestingly, this same pattern of brain activity can be elicited by physical challenges, including exercise stress,41 cold-pressor tests,42 inflammatory challenges,43 and experienced pain,44 by psychological challenges, including mental stress,41,45 and by “social” challenges that include rejection by others46 or perceiving another person to be in pain.47 While there may be modality-specific differences in the patterns of brain activity produced by these different stressors, the commonalities described seem to represent a signature of physiological and often psychological arousal challenging the individual,48,49 and are subsumable under the concept of emotional or behavioral salience.50 Fear is thus only one example of the engagement of this system. Amygdala activation is often assumed to be a definitive signature of fear processing, yet is observed in only a proportion of fear studies,51 and as a component of the proposed salience network can occur with nonfear demanding tasks and arousing stimuli.52,53
Fear learning and extinction
Fear is learned rapidly through a basic associative mechanism. Pavlovian fear conditioning describes the pairing of a stimulus (CS) with an aversive unconditioned stimulus (US) to induce a fear reaction known in this context as the conditioned response (CR). The CS becomes predictive of the occurrence of the US, and thus gains emotional salience as a conditioned threat cue (CS+), able on its own to induce a fear reaction/CR. Repeated presentations of the CS+ in the absence of the aversive US can lead to “extinction” of the capacity of the CS to induce the CR. Extinction learning does not erase the fear memory, but rather creates a new inhibitory safety memory,54 which may be confined to a particular context. Delayed testing can explore extinction retention, indicating whether extinction memory prevails with the passage of time or whether the initial fear memory is now expressed.
Human neuroimaging experiments emphasize the role of the amygdala in the establishment of conditioned fear. This is in keeping with fear-learning mechanisms defined in nonhuman animals.55 Such research has progressed to understanding neural processing and temporal dynamics involved in assigning, reassigning, and predicting value to stimuli, often within established learning theory models.19,21–23 Reviews and meta-analyses of human neuroimaging studies of classic Pavlovian fear conditioning highlight the engagement of the amygdala along with the insula and cingulate cortices.56,57 Additional engagement of the hippocampus occurs when there is a delay between the threat and predicted aversive outcome (trace conditioning). In conditioning experiments, the amplitude of amygdaloid (and hippocampal) responses to conditioned threat stimuli appears to decrease over time with repeated presentations.23,56 This is consistent with a consolidation of initial associative processing within the medial temporal lobe into representations elsewhere in the brain (striatal and cortical regions).56,57 A number of neuroimaging studies that did not show strong amygdala involvement in fear conditioning did not test for time-dependent effects either.57 It should be noted, however, that the habituation of amygdala responses is not limited to conditioned stimuli, but appears more generalizable and occurs in other classes of emotional stimuli, including emotional faces.58 The amygdala is sensitive to salience and novelty,59 and here the notion of neophobia is important. With repeated stimulus presentations (and better perceptual and affective characterization) amygdala activation is dampened over time. Regional brain activity during conditioned fear also maps changes in physiological arousal: activation of regions that include the amygdala and insula are sensitive to feedback of autonomic response.60 The response within the right mid- and anterior insula furthermore reflects the conjunction of autonomic response and conscious awareness of the threat, consistent with some “constructionist” models of emotion.9,61
Fear extinction is a form of new learning that results in the inhibition of conditioned fear, where trait deficits in fear extinction are a risk factor for anxiety disorders. It is important for a fear system to learn adaptively that something that was once feared is no longer a threat. The prefrontal cortex has a key role in the process of extinction: animal studies specifically implicate the infralimbic cortex.54,62 In humans, neuroimaging suggests a human homologue within the subgenual anterior cingulate and adjacent ventromedial prefrontal cortex. This region interacts with amygdala reactivity to suppress fear responses across a number of contexts.19,63 Interestingly, activity within this area correlates inversely with the tonic state of sympathetic arousal.64 The hippocampus also contributes to fear extinction, influencing the strength of safety memory and its recollections, thereby extending the suppression of fear reactions beyond the immediate situation.63,65 Contextual information is critical for interpreting ambiguous cues, modulating expression of stimulus–response contingencies when cue meanings depend on specific environments.65 When fear acquisition and extinction occur in different contexts, fear returns when a CS+ is reintroduced in the acquisition context, a phenomenon known as fear renewal.66 In contrast, when the extinguished CS is reintroduced in the extinction context, it does not elicit fear, demonstrating extinction recall. The acquisition environment thus represents a “danger” context, and the extinction environment a “safety” context.
Fear and anticipation
Fear conditioning exemplifies a more general principle of fear as an anticipatory emotion: beyond delay-conditioning studies (in which there is immediate learning that a specific cue signals an aversive event), fear and anxiety can be robustly elicited using “anticipation of shock” paradigms in which a context, eg, a colored screen, probabilistically signals the likelihood of receiving a “random” shock. These types of studies are better experimental models of anxiety, encapsulating how a generalized expectation of something aversive underpins the expression of anxiety disorders, including panic. Speculatively, the fact that one is intrinsically unable to identify a specific object cue ramps up the arousal and sensory attentional processes to fuel the fear state and enhance the salience of interoceptive cues (eg, sensations of autonomic arousal) and memory of prior aversive (shock) stimuli, as might occur in fear-potentiated startle in anxious individuals.67 Trace (unlike delay)-conditioning protocols embody an anticipatory interval before an aversive outcome: here, the bodily (electrodermal) and subjective expression of anxiety ramps up with the proximity and signaled intensity of shock, mirroring the linear ramping of activity within the insula, anterior cingulate cortex, and inferior frontal gyrus.68 These findings highlight the tuning of psychophysiological arousal and subjective emotional responses during fear anticipation to the activity within a tight network of affective and regulatory paralimbic and prefrontal cortices, without obligatory amygdala engagement.69
Communication of fear signals
The majority of neuroimaging studies of emotion (including fear) use face stimuli depicting emotional expressions to activate fear centers in the scanned observer.70 Implicit to this approach is the notions of emotion contagion, social cognition, and empathy that were explored, where the reactivity of amygdala to fear faces predicts social cognitive functioning in healthy individuals and clinical populations (eg, autism).71,72 Across studies, it has been found that fear faces relative to other emotional facial expressions are associated with enhanced amygdala responses.49,63 This observation remains largely true, even for parts of faces (fear eyes) and subliminal processing of such fear signals.73,74 However, it is important to acknowledge, as illustrated empirically, that the amygdala will respond more generally to face stimuli, to emotional information, and to the meaning, salience, and arousal contained within the faces.53 Fear faces (and vocalizations) are often more emotive, salient, and arousing than other emotional expressions. Therefore, the specificity of the fear circuitry and amygdala responses to fear faces may be in part confounded by generic qualities of these stimuli. Consistent with this view is the observation that amygdala responses correlate with ratings of emotional arousal in face stimuli irrespective of the type and valence of the depicted emotion.51,53 The incidental and predicted physiological arousal state of the individual may also contribute to such responses.75,76
Body and posture
Bodily expressions are also important to the communication of fear, yet are often overlooked relative to facial signals of emotion. Observed posture can communicate the emotional state (and implied risk to others) from a much greater distance than facial expression. Neuroimaging studies of the perception of fear signals from static or dynamic bodily postures show the engagement of the amygdala and insula to fear and anger bodily signals, along with visuomotor and somatosensory regions encoding movements and their motivational meanings.77,78
Music and fear
Fear is typically considered a low-level basic emotion, transmittable between individuals involuntarily through fast perceptual contagion, yet as with other emotions, through music fear can also reach a level of cognitive, interpersonal, and cultural sophistication. Music can be a powerful means to evoke fear, and will amplify the processing of cues of threat and menace from other modalities (much used by the film industry). Fearful music may attenuate the extent and amplitude of auditory activity (most activated by joyful music). This effect is associated with decreased activity within regions of the (superficial) amygdala associated with motivation and action through dense anatomical connections to the ventral striatum. However, when fearful visual object cues are viewed, the activity within the basolateral amygdala is augmented by congruent fear-evoking music.78 On its own, fear-evoking music increases functional connectivity between the auditory cortex, insula, and anterior cingulate,79 where the processing of musical cues of fear overlap with responses to fear vocalizations.80 Therefore, through music, fear may be conveyed both as a context and cue. However, the ability to recognize fear within music appears to be universal.81,82
Fear, awareness, and attention
Fear stimuli grab attention, and perhaps none more so than fear signals from other people. People orientate to potential threat, even if the threat signal is degraded or ambiguous, leading to shifts in spatial attention and the size of the attentional window. Stimuli presented at the limits of perceptual detection (eg, in an attentional blink or partial masking paradigm) are more likely to break through to conscious awareness if they convey a fearful facial expression or are threat-related. Memory is enhanced for threatening items, and for items processed in a threatening context. Human functional brain imaging has enabled identification of the neural substrates through which sensory processing and attention are modulated by threat stimuli. Again, the amygdala is implicated in both direct and indirect top-down enhancement of earlier sensory processing to influence the representation of threat.83
Subliminal detection of fear
Importantly for survival value, the detection of fear signals can occur subliminally, evoking covert motoric and autonomic responses and often behavioral response tendencies in the absence of evidence for conscious processing. Neuroimaging has helped in the understanding of underlying neurobiological processes. The amygdala responds to masked (“unseen”) emotional stimuli.21,24,74 Based on animal experiments, a subcortical route to the amygdala for rapid preconscious processing of threat is supported by neuroimaging findings, including studies of patients with “blindsight”. These individuals are blind in sectors of their visual field following damage to the primary visual cortex. Nevertheless, it can be shown that they can process information from fear faces presented within the visual blind area.83 When fear faces are presented subliminally, there is increased functional connectivity between the right amygdala, pulvinar, and superior colliculus.21,24 This subcortical pathway provides a fast subcortical route to the amygdala for the rough and ready but evolutionarily advantageous rapid detection of threat, triggering automatic defensive reactions.55
Conscious detection of threat
It is clearly advantageous for conscious attention to be drawn to threat. The capturing of attention by fear stimuli is evident in findings that show increased detection of threat-related stimuli presented at the limits of conscious perception. The emotional attentional blink task is a useful illustration of this: if two target stimuli are embedded within a series of rapidly presented distractor stimuli, the detection of the second target is impaired by presentation of the first target in the region of 200–350 ms earlier. This is known as the attentional blink, describing a time window within which attentional resources are relatively impoverished. Emotional stimuli, notably fear faces, when presented as this second target can “break through” to be detected more than nonemotional stimuli. This effect is referred to as the emotional attentional blink. Functional neuroimaging demonstrates enhanced activity within the fusiform cortex for stimuli that break through. Also, enhanced activity within the rostral anterior cingulate cortex84 and amygdala85 can account for the particular benefit in the detection of fearful stimuli. Functional connectivity between the amygdala and visual cortex is also enhanced with fear stimuli, as demonstrated using fear-evoking music,80 providing a potential additional mechanism through which fear can induce increased visual alertness and orientating of attention.
Spatial attention and threat
Spatial attention is also drawn to potential threats: the combination of a fear-conditioning protocol and a target-detection task has been used to test how threat signals that capture an individual’s spatial attention can lead to more efficient detection of and faster responses to events occurring in that part of the environment. While conditioned fear stimuli enhance activity within the amygdala and extrastriate visual cortex, the modulation of spatial attention by fear is mediated by the lateral frontoparietal cortices (implicated in spatial attention) and changes in the activity of the lateral orbitofrontal cortex.86 Within early visual cortices, the facilitation by fear of the detection of targets in particular spatial locations is demonstrated using electroencephalography (EEG) in which covert orientating due to fear stimuli selectively increases an early positive (P1) potential from a lateral occipital/extrastriate source.87 The very earliest electroencephalography of visual processing in component 1 (C1) from the striate cortex is amplified in fearful individuals in response to both fear-eliciting (eg, spider) and nonfearful stimuli (eg, flowers).88 Moreover, when fear faces are used in a task requiring detection of spatial targets, there is a selective modulation of the intraparietal and orbitofrontal cortex when fear stimuli distract attention away from the spatial location of the target, yet for the same location, occipital cortex activity is enhanced.89 These observations suggest two partially dissociable mechanisms through which fear signals influence spatial attention by 1) disengaging parietal spatial attention to alternative locations, and 2) increasing sensory processing within visual cortical representation of the fear location.
Contextual influences on fear
The magnitude and expression of responses to threat is highly dependent on context, which may be internal to the individual (psychological and physiological state, prior experience),90 external, ie, related to the environment (proximity and setting of the threat and the social context), or the conjunction of a threat with other moderating cues: we feel less fear when we feel stronger, protected and reassured (eg, by the knowledge that the nearby snake is in a cage). This dependence of fear responses on context is important, not least because it qualifies the notion of fear as an automatic, universal, defensive emotion. Recognition of context effects also enriches our understanding of the genesis and potential management of such psychological disorders as phobia, fear, and panic.
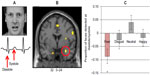
Fear reactions, as defensive behaviors, are attuned to the context of threat. The expression of fear changes in proportion to the proximity of threat. In predated animals, there is gradated behavioral switching along a continuum of proximity from “pre-encounter” (no immediate danger), via encounter (imminent danger), to “circa strike”, where danger is unavoidable. This “predatory imminence continuum” is proposed to modulate consequent fight, flight, or freeze behaviors.91 One elegant neuroimaging study used a maze-based video game that required players to avoid “predators” where capture was associated with electrocutaneous shocks.92 The study found that the shift toward imminent danger was instantiated within the brain by a shift in activity from the rostral and ventromedial prefrontal cortex and basolateral amygdala (arguably engaged in evaluative processing) toward the central amygdala nucleus and midbrain periaqueductal gray matter (more closely coupled to bodily response). Interestingly, periaqueductal gray-matter activity correlated with the dread elicited by increasing proximity of threat. This set of findings reconciles a number of discrepancies within the fear literature (including, eg, distinct cardiac responses to threat at different physical proximities), and bridges human and animal literature (see Figure 1).93
Body posture and fear
As noted, distant fear signals are conveyed by the posture and bodily actions (facial expressions are more proximate signals), are rapidly processed, and lead to contagion of similar postures that ready an individual for rapid escape. The signals from bodily posture can override or reframe the interpretation of signals from facial expressions, such that up to a 30% shift in the rating of “basic” emotional facial expressions can occur if, eg, a fearful face is placed on an angry body or vice versa.94,95 An individual’s own posture and physiological state also influences sensitivity to emotive cues and shapes the emotional response: holding a facial expression of emotion influences the judgment of emotional facial expressions of others,96 and this effect is associated with differences in the engagement of brain regions, including the amygdala, superior temporal sulcus, and insula. Bodily posture, eg, leaning forward or reclining, has a similar impact on one’s responses to and appraisal of emotive material.97 This may be an important consideration when generalizing inferences from neuroimaging experiments, given the constraint upon participant posture and action within scanning environments.
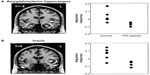
Posture and action also dynamically influence the physiological state of the body. Internal states of arousal exert a major influence on fear processing. A state of exaggerated physiological arousal is associated with increased fear reactivity, particularly in such clinical conditions as anxiety disorders. The neural mechanisms through which this interoceptive context influences fear processing can be explored in a number of ways using neuroimaging, eg, increasing heart rate and blood pressure using drugs or incidental tasks. One neat approach is to exploit the cardiac cycle.98 The strength and timing of individual heartbeats is conveyed to the brain by baroreceptor activity: each time the heart ejects blood, a signal from arterial baroreceptors in the great vessels is transmitted centrally. Brief experimental stimuli can be presented during this baroreceptor signal (on the heartbeat) or between heartbeats, when baroreceptors are quiet. The baroreceptor signal is used in the reflex control of blood pressure (eg, with posture), and was previously thought to have a general inhibitory effect on sensory attentional processes, including pain. Recently, Garfinkel et al75 overturned this generalized view to show that fear processing is enhanced by baroreceptor activation. There is better detection of periliminal fear faces (in an emotional attentional blink task) and augmentation of fear ratings of overt fear faces when these stimuli are presented on the heartbeat (at systole). The neural responsivity of such regions as the amygdala mediates this dependence of fear processing on heartbeat timing (see Figure 2). These findings highlight the contribution of physiological signals from the body to the processing of fear stimuli, effects previously noted in fear-conditioning studies of patients with autonomic failure (see Figure 3).60
Insights from pharmacological and genetic neuroimaging
Neuroimaging studies of the pharmacological/neurochemical modulation of fear responses and of individual differences (genetic or otherwise) in fear processing typically link changes in the experience of fear to measured differences in amygdala reactivity. As noted earlier (“Fear learning and extinction” section), amygdala responses to threat stimuli change with time over the course of an experiment. Therefore, reports of “enhanced” amygdala reactivity may reflect more complex expression of time-dependent effects. Classic anxiolytic medication, such as the benzodiazepine diazepam, attenuates amygdala responses to angry and fear faces. This is associated with reduced anxiety and also accompanied by reduced orbitofrontal cortex activation. Interestingly, effects on anterior cingulate function are more complex, with different effects on responses to angry and fear faces.99 Some other pharmacological agents show sex-specific effects: for example, in men, oxytocin decreases amygdala reactivity to aversive, threat-related scenes and fearful, threat-related faces,100,101 but in women elicits increases in amygdala reactivity to similar stimuli.102,103 The effect, at least in men, may be potentially useful in mitigating or attenuating the expression of PTSD.104
The efficacy of particular pharmaceutical agents in the treatment of clinical anxiety has directed exploration of candidate genes and common variants in receptors and transporter molecules linked to affective vulnerability. Examples include the relationship between PTSD and 5-hydroxytryptamine (5-HT, serotonin) transporter SERT. Common SERT genetic polymorphisms within the promoter region (such that there is a long [L] allele and a short [S] allele) influence interindividual differences in the expression of response to traumatic events.105 SERT expression at the presynaptic membrane and 5-HT-uptake activity are significantly greater in carriers of the L compared to the S allele.106 The specificity to fear- and anxiety-related responses is weak however, yet interactions between the 5-HT polymorphism with early stressful life events influence vulnerability factors to depressive illnesses105 and anxiety sensitivity. Similar common polymorphisms are present in the COMT gene. COMT is an enzyme responsible for the removal of monoamine neuromodulators, including dopamine and noradrenaline. The met (methionine) variant, compared to the val (valine) variant of COMT, leads to slower monoamine degradation, and is associated with improved functioning on some cognitive domains, differences in vulnerability to affective and anxiety disorders, and enhanced amygdala responses to negative visual stimuli. Amygdala reactivity to fear stimuli is proposed as a heritable trait, linked to both the expression of anxiety and depression.107 Both the SERTlong and the COMTmet polymorphisms enhance this reactivity, likely increasing sensitivity to detect biologically and socially relevant information.108
Other polymorphisms overrepresented in anxiety and panic populations and linked to enhanced amygdala responsivity to threat stimuli include neuropeptide S109 and pituitary adenylate cyclase-activating polypeptide-receptor genes.110 There is also interest in polymorphisms of BDNF,111,112 which appear to exert a constrained effect on extinction learning, and could thus represent a further target for personalized treatment of anxiety symptoms. Anxiety disorders vary in the degree to which there is familial loading and genetic vulnerability. Heritability is commonly observed in obsessive compulsive disorder for select “vulnerability” subgroups, eg, people with blood-phobia syncope, including systemic conditions (eg, joint hypermobility syndrome).113 Anxiety is however a pervasive symptom across different psychiatric disorders, including persecutory ideation in people with psychosis and social fears expressed in autism-spectrum conditions. Nevertheless, the genetics of “pure” anxiety disorder are illustrated by twin studies, analyses of specific phenotypes, and at the population level, facilitated now by access to genome-wide scanning.114
Anterior and posterior hippocampal formation show different cytoarchitectonic properties and distinct patterns for gene expression.115 Neuroimaging studies also reveal a contrast between the posterior hippocampus, as a substrate for predominantly spatial and cognitive (memory) processes, and the anterior hippocampus, which is implicated in emotional processes, particularly in regard to negative affect, fear, and stress.115,116
Clinical expressions: phobia and posttraumatic stress disorder
Specific phobias
In patients with specific phobias, neuroimaging has been applied to understand the neural substrates underlying the psychopathology and reveal potential novel pathways for intervention. Different types of phobia are important to consider here, notably a distinction between blood phobias (including needle and body boundary-violation phobia) and other specific phobias, eg, spiders or other animals. Social phobia is more related to general anxiety disorder and other less specific anxiety conditions. Blood and needle phobia are closely coupled to emotional “simple” fainting (neurocardiogenic syncope). Structural anatomical differences in the brain stem are associated with a vulnerability to simple faints too, while the local volume of neighboring sectors of the caudate nucleus correlate with parasympathetic tone and trait anxiety, and where these regions overlap, predict fainting frequency.117 Across specific phobias, functional imaging studies report enhanced activation of the amygdala (and/or adjacent globus pallidus), insula, thalamus, and cerebellum to phobic stimuli.118,119 These findings are in keeping with an exaggerated sensitivity to particular stimuli as salient threats, rather than pointing to a core neurobiological abnormality.
Posttraumatic stress disorder
Similar conclusions come from a meta-analysis searching for functional neurobiological signatures of anxiety disorders that made direct comparison to brain correlates of anticipatory anxiety induced using fear conditioning relative to healthy individuals.120 Patients with PTSD, social anxiety disorder, or specific phobia share the enhanced activation of the amygdala and insula, in a similar pattern to the response of healthy individuals during conditioned fear. However, the pattern of exaggerated fear appears different in people with PTSD: in these patients, amygdala and insula responses are relatively attenuated compared to patients with phobia and social anxiety. Hypoactivation of the dorsal and rostral anterior cingulate and ventromedial prefrontal cortex are also observed in PTSD. Therefore, while there are common brain mechanisms in anxiety disorders and “normal” fear processing, there are additional features unique to the expression of PTSD that distinguish it from exaggerated fear.120
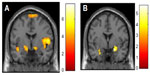
PTSD is associated with abnormalities in fear-associated learning, including greater acquisition of conditioned fear, overgeneralization of conditioning, impaired inhibitory learning, and impaired extinction.121–123 It is suggested that deficits in fear-associated learning play a role in the development and maintenance of PTSD.121,123 Abnormalities in the extinction and retention of conditioned fear are important to the persistence of fear memories in PTSD (see Figure 4).124,125 In PTSD, a pervasive sense of impending danger, fearfulness, and heightened arousal persists when no actual threat is present. This is thought to reflect an inability to modulate fear expression using contextual information.126 The amygdala, ventromedial prefrontal cortex, and hippocampus are all involved in fear-associated learning and contextual processing.65 Not only do PTSD patients manifest raised amygdala activity,127 suggesting enhanced fear-signal processing, they also show decreases in ventromedial prefrontal responsivity.128 This pattern is a basis of problems in emotion regulation and fear inhibition,129,130 contributing both to enhanced fear states and deficits in extinction (ie, safety) recall.124 Hippocampal function is also abnormal in PTSD,131 and there is also evidence of structural hippocampal deficits with reduced volume and neuronal integrity.132,133 Given the role of the amygdala, hippocampus, and ventromedial prefrontal cortex in context-dependent fear,134 this evidence supports more extensive examination of context processing in PTSD. It is suggested that PTSD patients fail to utilize safety signals.135 Fear memories that are not modulated by context can contribute to a persistent state of perceived threat and imminent danger, driving hyperarousal and avoidant behaviors. The flip side is that there may be a failure to respond appropriately to novel threats. This provides a neurobiological explanation for the seemingly counterintuitive observation that often PTSD patients are exposed to repeated traumas, potentially indicating a failure to recognize danger.136,137
Abnormal processing of trauma-related threat stimuli in PTSD patients is demonstrated by overattention to trauma-related cues,138 exaggerated physiological responses such as heart rate, skin conductance, electromyography, and blood pressure to trauma-cue exposure,122,139 and altered brain-activation patterns in symptom-provocation studies,140 yet PTSD patients display more general information-processing deficits. There is evidence that PTSD brains are attuned to preferentially detect and process threat material,141,142 show heightened orientating responses to novel stimuli,143 and may fail to filter out irrelevant sensory information.144 These deficits underlie the difficulty PTSD patients have in learning safety cues,145 and facilitate the reemergence of conditioned fear responses after extinction (Figure 4).146
Conclusion
What we are learning about fear at the neural and behavioral level in humans may be generalized to understanding the processes that shape our engagement with others and our willingness to pursue novel and potentially risky situations. While fear is a negative emotion, it shapes adaptive behavior, prudent decision making, and self-fulfillment through brave deeds and selfless actions. Human neuroimaging studies are largely constrained by their focus on the individual, underplaying the more social aspects of fear, eg, the way in which panic can spread through a crowd (or financial market). The processing of risk and threat appears stereotyped in automaticity and response repertoires, yet proves to be context-dependent and potentially either inhibits or facilitates action. There is redundancy within the fear system. Potential threats are inferred from partial information, as the cost of missing a real threat may be catastrophic. Neuroimaging teaches us how fear and risk closely interact with learning and memory to set emotional tone and response style at the individual level. The same themes play out at a societal level, where fear and prejudice can control populations, and catastrophic outcomes are repeated in different contexts with each generation.
Acknowledgments
The authors are grateful to Dr Neil Harrison for advice on the manuscript. HDC and SNG were supported by an Advanced Grant from the European Research Council to HDC and through the Dr Mortimer and Dame Theresa Sackler Foundation via the Sackler Centre for Consciousness Science, University of Sussex.
Disclosure
The authors report no conflicts of interest in this work.
References
Damasio A, Carvalho GB. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci. 2013;14:143–152. | |
Darwin C. The Expression of the Emotions in Man and Animals. London: John Murray; 1872. | |
Ekman P. Darwin and Facial Expression: A Century of Research in Review. New York: Academic; 1973. | |
Panksepp J. The basic emotional circuits of mammalian brains: do animals have affective lives? Neurosci Biobehav R. 2011;35:1791–1804. | |
Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. | |
Altenmuller E, Schmidt S, Zimmermann E, editors. Evolution of Emotional Communication: From Sounds in Nonhuman Mammals to Speech and Music in Man. Oxford: Oxford University Press; 2013. | |
Barrett LF. Solving the emotion paradox: categorization and the experience of emotion. Pers Soc Psychol Rev. 2006;10:20–46. | |
Barrett LF. Psychological construction: the Darwinian approach to the science of emotion. Emot Rev. 2013;5:379–389. | |
Gendron M, Barrett LF. Reconstructing the past: a century of ideas about emotion in psychology. Emot Rev. 2009;1:316–339. | |
James W. The physical basis of emotion [reprint]. Psychol Rev. 1994;101:205–210. | |
Harrison NA, Kreibig S, Critchley HD. Physiology of emotion responses in the brain. In: Vuilleumier P, Armony JL, editors. Handbook of Human Affective Neuroscience. Cambridge: Cambridge University Press; 2013. | |
Kreibig SD. Autonomic nervous system activity in emotion: a review. Biol Psychol. 2010;84:394–421. | |
Stemmler G, Heldmann M, Pauls CA, Scherer T. Constraints for emotion specificity in fear and anger: the context counts. Psychophysiology. 2001;38:275–291. | |
Rainville P, Bechara A, Naqvi N, Damasio AR. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int J Psychophysiol. 2006;61:5–18. | |
Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cogn Emot. 1998;12:191–220. | |
Ohman A, Hamm A, Hugdahl K. Cognition and the autonomic nervous system: orienting, anticipation and conditioning. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. New York: Cambridge University Press; 2000:533–575. | |
Etzel JA, Johnsen EL, Dickerson J, Tranel D, Adolphs R. Cardiovascular and respiratory responses during musical mood induction. Int J Psychophysiol. 2006;61:57–69. | |
Pruessner JC, Declovic K, Khalili-Mahani N, et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance Imaging studies. Biol Psychiatry. 2008;63:234–240. | |
LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. | |
Morris JS, Dolan RJ. Dissociable amygdala and orbitofrontal responses during reversal fear conditioning. Neuroimage. 2004;22: 372–380. | |
Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci U S A. 1999;96:1680–1685. | |
Williams LM, Phillips ML, Brammer MJ, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14: 1070–1079. | |
Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. | |
Williams LM, Liddell BJ, Kemp AH, et al. Amygdala-prefrontal dissociation of subliminal and supraliminal fear. Hum Brain Mapp. 2006;27:652–661. | |
Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20:3033–3040. | |
Dimberg U. Facial reactions to fear-relevant stimuli for subjects high and low in specific fear. Scand J Psychol. 1990;31:65–69. | |
Schmidt NB, Richey JA, Zvolensky MJ, Maner JK. Exploring human freeze responses to a threat stressor. J Behav Ther Exp Psychiatry. 2008;39:292–304. | |
Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;97:377–395. | |
Pissiota A, Frans O, Michelgard A, et al. Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. Eur J Neurosci. 2003;18:1325–1331. | |
Mujica-Parodi LR, Strey HH, Frederick B, et al. Chemosensory cues to conspecific emotional stress activate amygdala in humans. Plos One. 2009;4:e6415. | |
Brannan S, Liotti M, Egan G, et al. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci U S A. 2001;98:2029–2034. | |
Corfield DR, Fink GR, Ramsay SC, et al. Evidence for limbic system activation during CO2-stimulated breathing in man. J Physiol. 1995;488:77–84. | |
Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88:1500–1511. | |
Feinstein JS, Buzza C, Hurlemann R, et al. Fear and panic in humans with bilateral amygdala damage. Nat Neurosci. 2013;16:270–272. | |
Eser D, Leicht G, Lutz J, et al. Functional neuroanatomy of CCK-4-induced panic attacks in healthy volunteers. Hum Brain Mapp. 2009;30:511–522. | |
Schunck T, Erb G, Mathis A, et al. Functional magnetic resonance imaging characterization of CCK-4-induced panic attack and subsequent anticipatory anxiety. Neuroimage. 2006;31:1197–1208. | |
Schunck T, Mathis A, Erb G, Namer IJ, Demazieres A, Luthringer R. Effects of lorazepam on brain activity pattern during an anxiety symptom provocation challenge. J Psychopharmacol. 2010;24:701–708. | |
Dedovic K, Rexroth M, Wolff E, et al. Neural correlates of processing stressful information: an event-related fMRI study. Brain Res. 2009;1293:49–60. | |
Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. | |
Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat: part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. | |
Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol. 2000;523:259–270. | |
Frankenstein UN, Richter W, McIntyre MC, Remy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage. 2001;14:827–836. | |
Harrison NA, Brydon L, Walker C, et al. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66:415–422. | |
Tracey I, Becerra L, Chang I, et al. Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2000;288: 159–162. | |
Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–999. | |
Eisenberger NI, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7:745–754. | |
Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. | |
Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol. 2009;73:88–94. | |
Critchley HD, Nagai Y, Gray MA, Mathias CJ. Dissecting axes of autonomic control in humans: insights from neuroimaging. Auton Neurosci. 2011;161:34–42. | |
Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. | |
Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. | |
Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflex suppression during stress in humans. Hum Brain Mapp. 2012;33:1700–1716. | |
Winston JS, O’Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20:84–97. | |
Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. | |
LeDoux JE. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York: Simon and Schuster; 1998. | |
Buchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10:219–223. | |
Sehlmeyer C, Schöning S, Zwitserlood P, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. Plos One. 2009;4:e5865. | |
Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. | |
Knutson B, Cooper JC. The lure of the unknown. Neuron. 2006;51: 280–282. | |
Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. | |
Schachter S, Singer JE. Cognitive, social, and physiological determinants of emotional state. Psychol Rev. 1962;69:379–399. | |
Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. | |
Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62: 446–454. | |
Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage. 2004;22:243–251. | |
Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. | |
Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. | |
Melzig CA, Michalowski JM, Holtz K, Hamm AO. Anticipation of interoceptive threat in highly anxiety sensitive persons. Behav Res Ther. 2008;46:1126–1134. | |
Drabant EM, Kuo JR, Ramel W, et al. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage. 2011;55:401–410. | |
Mee S, Bunney BG, Reist C, Potkin SG, Bunney WE. Psychological pain: a review of evidence. J Psychiatr Res. 2006;40:680–690. | |
Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. | |
Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. | |
Corden B, Critchley HD, Skuse D, Dolan RJ. Fear recognition ability predicts differences in social cognitive and neural functioning in men. J Cogn Neurosci. 2006;18:889–897. | |
Morris JS, deBonis M, Dolan RJ. Human amygdala responses to fearful eyes. Neuroimage. 2002;17:214–222. | |
Whalen PJ, Kagan J, Cook RG, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. | |
Garfinkel SN, Minati L, Gray MA, Seth AK, Dolan RJ, Critchley HD. Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. J Neurosci. 2014;34:6573–6582. | |
Gray MA, Harrison NA, Wiens S, Critchley HD. Modulation of emotional appraisal by false physiological feedback during fMRI. Plos One. 2007;2:e546. | |
Kret ME, Pichon S, Grèzes J, de Gelder B. Similarities and differences in perceiving threat from dynamic faces and bodies. An fMRI study. Neuroimage. 2011;54:1755–1762. | |
Pichon S, de Gelder B, Grèzes J. Two different faces of threat. Comparing the neural systems for recognizing fear and anger in dynamic body expressions. Neuroimage. 2009;47:1873–1883. | |
Baumgartner T, Lutz K, Schmidt CF, JÄncke L. The emotional power of music: how music enhances the feeling of affective pictures. Brain Res. 2006;1075:151–164. | |
Koelsch S, Skouras S, Fritz T, et al. The roles of superficial amygdala and auditory cortex in music-evoked fear and joy. Neuroimage. 2013;81:49–60. | |
Juslin PN, Laukka P. Communication of emotions in vocal expression and music performance: different channels, same code? Psychol Bull. 2003;129:770–814. | |
Fritz T, Jentschke S, Gosselin N, et al. Universal recognition of three basic emotions in music. Curr Biol. 2009;19:573–576. | |
Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9:585–594. | |
De Martino B, Kalisch R, Rees G, Dolan RJ. Enhanced processing of threat stimuli under limited attentional resources. Cereb Cortex. 2009;19:127–133. | |
Schwabe L, Merz CJ, Walter B, Vaitl D, Wolf OT, Stark R. Emotional modulation of the attentional blink: the neural structures involved in capturing and holding attention. Neuropsychologia. 2011;49: 416–425. | |
Armony JL, Dolan RJ. Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia. 2002;40:817–826. | |
Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cereb Cortex. 2004;14:619–633. | |
Weymar M, Keil A, Hamm AO. Timing the fearful brain: unspecific hypervigilance and spatial attention in early visual perception. Soc Cogn Affect Neurosci. 2014;9:723–729. | |
Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. Neural systems for orienting attention to the location of threat signals: an event-related fMRI study. Neuroimage. 2006;31:920–933. | |
LeDoux JE. Coming to terms with fear. Proc Natl Acad Sci U S A. 2014;111:2871–2878. | |
Blanchard RJ, Blanchard DC. Anti-predator defence as models of fear and anxiety. In: Brain PF, Parmigiani S, Blanchard RJ, Mainardi D, editors. Fear and Defence. Amsterdam: Harwood Academic; 1990:89–108. | |
Mobbs D, Petrovic P, Marchant JL, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. | |
Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1:429–438. | |
Aviezer H, Trope Y, Todorov A. Body cues, not facial expressions, discriminate between intense positive and negative emotions. Science. 2012;338:1225–1229. | |
de Gelder B. Towards the neurobiology of emotional body language. Nat Rev Neurosci. 2006;7:242–249. | |
Lee TW, Dolan RJ, Critchley HD. Controlling emotional expression: behavioral and neural correlates of nonimitative emotional responses. Cereb Cortex. 2008;18:104–113. | |
Price TF, Dieckman LW, Harmon-Jones E. Embodying approach motivation: body posture influences startle eyeblink and event-related potential responses to appetitive stimuli. Biol Psychol. 2012;90: 211–217. | |
Gray MA, Beacher FD, Minati L, et al. Emotional appraisal is influenced by cardiac afferent information. Emotion. 2012;12:180–191. | |
Del-Ben CM, Ferreira CA, Sanchez TA, et al. Effects of diazepam on BOLD activation during the processing of aversive faces. J Psychopharmacol. 2012;26:443–451. | |
Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. | |
Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. | |
Domes G, Lischke A, Berger C, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. | |
Lischke A, Gamer M, Berger C, et al. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37:1431–1438. | |
Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: salience processing and fear inhibition processes. Psychoneuroendocrinology. 2014;40:242–256. | |
Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. | |
Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism – basic research and clinical implications. J Neural Transm. 1997;104:1005–1014. | |
Lonsdorf TB, Golkar A, Lindstöm KM, et al. 5-HTTLPR and COMTval158met genotype gate amygdala reactivity and habituation. Biol Psychol. 2011;87:106–112. | |
Domschke K, Baune BT, Havlik L, et al. Catechol-O-methyltransferase gene variation: impact on amygdala response to aversive stimuli. Neuroimage. 2012;60:2222–2229. | |
Dannlowski U, Kugel H, Franke F, et al. Neuropeptide-S (NPS) receptor genotype modulates basolateral amygdala responsiveness to aversive stimuli. Neuropsychopharmacology. 2011;36:1879–1885. | |
Stevens JS, Almli LM, Fani N, et al. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proc Natl Acad Sci U S A. 2014;111:3158–3163. | |
Frielingsdorf H, Bath KG, Soliman F, DiFede J, Casey BJ, Lee FS. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann N Y Acad Sci. 2010;1208:150–157. | |
Lonsdorf TB, Weike AI, Golkar A, Schalling M, Hamm AO, Ohman A. Amygdala-dependent fear conditioning in humans is modulated by the BDNFval66met polymorphism. Behav Neurosci. 2010;124:9–15. | |
Eccles JA, Beacher FD, Gray MA, et al. Brain structure and joint hypermobility: relevance to the expression of psychiatric symptoms. Br J Psychiatry. 2012;200:508–509. | |
Smoller JW, Gardner-Schuster E, Covino J. The genetic basis of panic and phobic anxiety disorders. Am J Med Genet C Semin Med Genet. 2008;148C:118–126. | |
Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. | |
Koelsch S. Brain correlates of music-evoked emotions. Nat Rev Neurosci. 2014;15:170–180. | |
Beacher FD, Gray MA, Mathias CJ, Critchley HD. Vulnerability to simple faints is predicted by regional differences in brain anatomy. Neuroimage. 2009;47:937–945. | |
Birbaumer N, Grodd W, Diedrich O, et al. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9: 1223–1226. | |
Ipser JC, Singh L, Stein DJ. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin Neurosci. 2013;67:311–322. | |
Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. | |
Lommen MJ, Engelhard IM, Sijbrandij M, van den Hout MA, Hermans D. Pre-trauma individual differences in extinction learning predict posttraumatic stress. Behav Res Ther. 2013;51:63–67. | |
Orr SP, Roth WT. Psychophysiological assessment: clinical applications for PTSD. J Affect Disord. 2000;61:225–240. | |
Sijbrandij M, Engelhard IM, Lommen MJ, Leer A, Baas JM. Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD). J Psychiatr Res. 2013;47: 1991–1997. | |
Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. | |
Sripada RK, Sarah NG, Liberzon I. Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Front Hum Neurosci. 2013;7:672. | |
Rougemont-Bücking A, Linnman C, Zeffiro TA, et al. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther. 2011;17:227–236. | |
Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. | |
Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. | |
Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. | |
Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. | |
St Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J Psychiatr Res. 2011;45:630–637. | |
Brown S, Freeman T, Kimbrell T, Cardwell D, Komoroski R. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of former prisoners of war with and without posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 2003;15: 367–370. | |
De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52: 1066–1078. | |
Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. | |
Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. | |
Deliramich AN, Gray MJ. Changes in women’s sexual behavior following sexual assault. Behav Modif. 2008;32:611–621. | |
Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112–1121. | |
Foa EB, Feske U, Murdock TB, Kozak MJ, Mccarthy PR. Processing of threat-related information in rape victims. J Abnorm Psychol. 1991;100:156–162. | |
Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133:725–746. | |
Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. | |
El Khoury-Malhame M, Reynaud E, Soriano A, et al. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49:1969–1973. | |
Fani N, Jovanovic T, Ely TD, et al. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol. 2012;90: 134–142. | |
Kimble M, Kaloupek D, Kaufman M, Deldin P. Stimulus novelty differentially affects attentional allocation in PTSD. Biol Psychiatry. 2000;47:880–890. | |
Mcfarlane AC, Weber DL, Clark CR. Abnormal stimulus processing in posttraumatic stress disorder. Biol Psychiatry. 1993;34:311–320. | |
Christianson JP, Fernando AB, Kazama AM, Jovanovic T, Ostroff LE, Sangha S. Inhibition of fear by learned safety signals: a mini-symposium review. J Neurosci. 2012;32:14118–14124. | |
Garfinkel SN, Abelson JL, King A, et al. Impaired contextual modulation of memories in PTSD: An fMRI and psychophysiological study of extinction retention and fear renewal. Journal of Neuroscience. 2014;40:13435–13443. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.